Page 36 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 36
the integration of biotransformations into catalyst 19
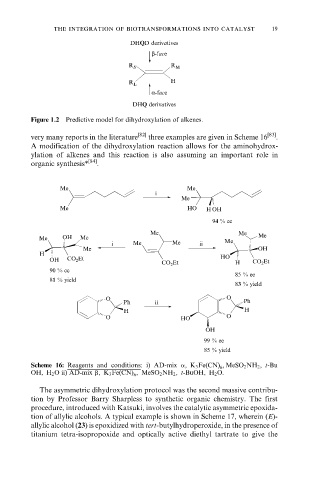
DHQD derivatives
β-face
R S R M
H
R L
α-face
DHQ derivatives
Figure 1.2 Predictive model for dihydroxylation of alkenes.
very many reports in the literature [82] three examples are given in Scheme 16 [83] .
A modification of the dihydroxylation reaction allows for the aminohydrox-
ylation of alkenes and this reaction is also assuming an important role in
organic synthesis* [84] .
Me Me
i
Me
Me HO H OH
94 % ee
Me Me
Me OH Me Me Me
i Me Me ii
Me OH
H HO
OH CO 2 Et CO 2 Et
CO 2 Et H
90 % ee
85 % ee
81 % yield
83 % yield
O O
Ph ii Ph
H H
O HO O
OH
99 % ee
85 % yield
Scheme 16: Reagents and conditions: i) AD-mix a, K 3 Fe(CN) , MeSO 2 NH 2 , t-Bu
6
OH, H 2 O ii) AD-mix b, K 3 Fe(CN) , MeSO 2 NH 2 , t-BuOH, H 2 O.
6
The asymmetric dihydroxylation protocol was the second massive contribu-
tion by Professor Barry Sharpless to synthetic organic chemistry. The first
procedure, introduced with Katsuki, involves the catalytic asymmetric epoxida-
tion of allylic alcohols. A typical example is shown in Scheme 17, wherein (E)-
allylic alcohol (23) is epoxidized with tert-butylhydroperoxide, in the presence of
titanium tetra-isopropoxide and optically active diethyl tartrate to give the