Page 32 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 32
the integration of biotransformations into catalyst 15
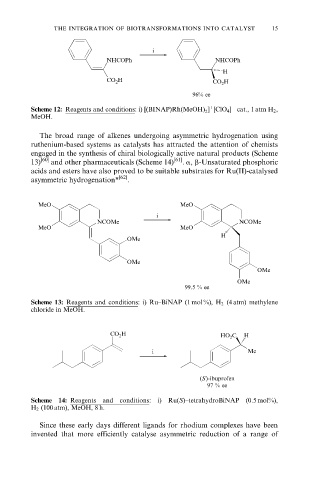
i
NHCOPh NHCOPh
H
CO 2 H CO 2 H
96% ee
ÿ
Scheme 12: Reagents and conditions: i) [(BINAP)Rh(MeOH) ] [ClO 4 ] cat., 1 atm H 2 ,
2
MeOH.
The broad range of alkenes undergoing asymmetric hydrogenation using
ruthenium-based systems as catalysts has attracted the attention of chemists
engaged in the synthesis of chiral biologically active natural products (Scheme
13) [60] and other pharmaceuticals (Scheme 14) [61] . a, b-Unsaturated phosphoric
acids and esters have also proved to be suitable substrates for Ru(II)-catalysed
asymmetric hydrogenation* [62] .
MeO MeO
i
NCOMe NCOMe
MeO MeO
H
OMe
OMe
OMe
OMe
99.5 % ee
Scheme 13: Reagents and conditions: i) Ru±BiNAP (1 mol %), H 2 (4 atm) methylene
chloride in MeOH.
CO 2 H HO 2 C H
i Me
(S )-ibuprofen
97 % ee
Scheme 14: Reagents and conditions: i) Ru(S)±tetrahydroBiNAP (0.5 mol%),
H 2 (100 atm), MeOH, 8 h.
Since these early days different ligands for rhodium complexes have been
invented that more efficiently catalyse asymmetric reduction of a range of