Page 27 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 27
10 hydrolysis, oxidation and reduction
1.2.1 REDUCTION OF CARBONYL COMPOUNDS
It is well known that bakers' yeast is capable of reducing a wide range of
ketones to optically active secondary alcohols. A recent example involves the
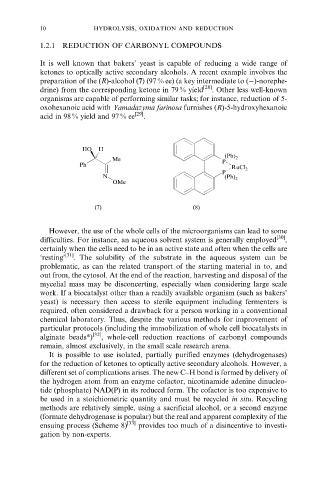
preparation of the (R)-alcohol (7) (97 % ee) (a key intermediate to (ÿ)-norephe-
drine) from the corresponding ketone in 79 % yield [28] . Other less well-known
organisms are capable of performing similar tasks; for instance, reduction of 5-
oxohexanoic acid with Yamadazyma farinosa furnishes (R)-5-hydroxyhexanoic
acid in 98 % yield and 97 % ee [29] .
HO H
Me P (Ph) 2
Ph
RuCl 2
P
N (Ph) 2
OMe
(7) (8)
However, the use of the whole cells of the microorganisms can lead to some
difficulties. For instance, an aqueous solvent system is generally employed [30] ,
certainly when the cells need to be in an active state and often when the cells are
`resting' [31] . The solubility of the substrate in the aqueous system can be
problematic, as can the related transport of the starting material in to, and
out from, the cytosol. At the end of the reaction, harvesting and disposal of the
mycelial mass may be disconcerting, especially when considering large scale
work. If a biocatalyst other than a readily available organism (such as bakers'
yeast) is necessary then access to sterile equipment including fermenters is
required, often considered a drawback for a person working in a conventional
chemical laboratory. Thus, despite the various methods for improvement of
particular protocols (including the immobilization of whole cell biocatalysts in
alginate beads*) [32] , whole-cell reduction reactions of carbonyl compounds
remain, almost exclusively, in the small scale research arena.
It is possible to use isolated, partially purified enzymes (dehydrogenases)
for the reduction of ketones to optically active secondary alcohols. However, a
different set of complications arises. The new C±H bond is formed by delivery of
the hydrogen atom from an enzyme cofactor, nicotinamide adenine dinucleo-
tide (phosphate) NAD(P) in its reduced form. The cofactor is too expensive to
be used in a stoichiometric quantity and must be recycled in situ. Recycling
methods are relatively simple, using a sacrificial alcohol, or a second enzyme
(formate dehydrogenase is popular) but the real and apparent complexity of the
ensuing process (Scheme 8) [33] provides too much of a disincentive to investi-
gation by non-experts.