Page 28 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 28
the integration of biotransformations into catalyst 11
i
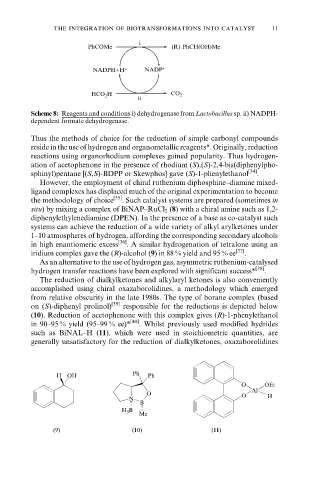
PhCOMe (R) PhCH(OH)Me
NADPH+H + NADP +
HCO 2 H CO 2
ii
Scheme 8: Reagents and conditions i) dehydrogenase from Lactobacillus sp. ii) NADPH-
dependent formate dehydrogenase.
Thus the methods of choice for the reduction of simple carbonyl compounds
reside in the use of hydrogen and organometallic reagents*. Originally, reduction
reactions using organorhodium complexes gained popularity. Thus hydrogen-
ation of acetophenone in the presence of rhodium (S),(S)-2,4-bis(diphenylpho-
sphinyl)pentane [(S,S)-BDPP or Skewphos] gave (S)-1-phenylethanol [34] .
However, the employment of chiral ruthenium diphosphine±diamine mixed-
ligand complexes has displaced much of the original experimentation to become
the methodology of choice [35] . Such catalyst systems are prepared (sometimes in
situ) by mixing a complex of BiNAP±RuCl 2 (8) with a chiral amine such as 1,2-
diphenylethylenediamine (DPEN). In the presence of a base as co-catalyst such
systems can achieve the reduction of a wide variety of alkyl arylketones under
1±10 atmospheres of hydrogen, affording the corresponding secondary alcohols
in high enantiomeric excess [36] . A similar hydrogenation of tetralone using an
iridium complex gave the (R)-alcohol (9) in 88 % yield and 95 % ee [37] .
As an alternative to the use of hydrogen gas, asymmetric ruthenium-catalysed
hydrogen transfer reactions have been explored with significant success* [38] .
The reduction of dialkylketones and alkylaryl ketones is also conveniently
accomplished using chiral oxazaborolidines, a methodology which emerged
from relative obscurity in the late 1980s. The type of borane complex (based
on (S)-diphenyl prolinol) [39] responsible for the reductions is depicted below
(10). Reduction of acetophenone with this complex gives (R)-1-phenylethanol
in 90±95 % yield (95±99 % ee)* [40] . Whilst previously used modified hydrides
such as BiNAL±H (11), which were used in stoichiometric quantities, are
generally unsatisfactory for the reduction of dialkylketones, oxazaborolidines
H OH Ph Ph
O OEt
Al
O O H
N
B
H 3 B
Me
(9) (10) (11)