Page 29 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 29
12 hydrolysis, oxidation and reduction
can be employed often with the production of secondary alcohols with high ee.
For example iso-propylmethyl ketone and tert-butylmethyl ketone are good
substrates giving secondary alcohols with > 91 % ee [41] . Alternatively oxaza-
phosphinamides* and hydroxysulfoximines* have been used to control the
stereochemistry of the reduction of simple ketones by borane.
Brook has effectively modified a procedure (introduced by Hosomi) which
employs a trialkoxysilane as the stoichiometric reducing agent which, in the
presence of amino acid anions reduces aryl alkyl ketones or diaryl ketones
to the corresponding (S)-secondary alcohols, albeit in modest ee (generally
25±40 %)*.
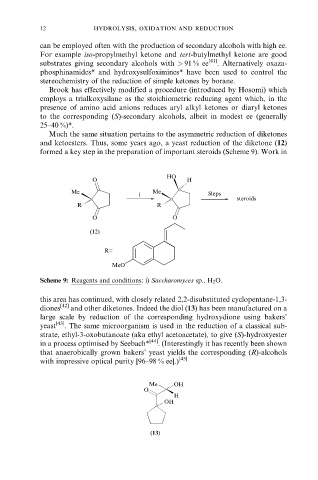
Much the same situation pertains to the asymmetric reduction of diketones
and ketoesters. Thus, some years ago, a yeast reduction of the diketone (12)
formed a key step in the preparation of important steroids (Scheme 9). Work in
HO
O H
Me i Me Steps
steroids
R R
O O
(12)
R=
MeO
Scheme 9: Reagents and conditions: i) Saccharomyces sp., H 2 O.
this area has continued, with closely related 2,2-disubstituted cyclopentane-1,3-
diones [42] and other diketones. Indeed the diol (13) has been manufactured on a
large scale by reduction of the corresponding hydroxydione using bakers'
yeast [43] . The same microorganism is used in the reduction of a classical sub-
strate, ethyl-3-oxobutanoate (aka ethyl acetoacetate), to give (S)-hydroxyester
in a process optimised by Seebach* [44] . (Interestingly it has recently been shown
that anaerobically grown bakers' yeast yields the corresponding (R)-alcohols
with impressive optical purity [96±98 % ee].) [45]
Me OH
O
H
OH
(13)