Page 30 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 30
the integration of biotransformations into catalyst 13
However, as for simpler carbonyl systems, organometallic catalysts offer a
powerful alternative to biotransformations. By way of comparison methyl-
3-oxobutanoate is reduced to the (R)-3-hydroxyester (> 99 % ee) quantitatively
using (R)-BiNAP±RuCl 2 under 100 atmospheres of hydrogen [46] . A variation of
this reaction using immobilized catalyst yields the chiral alcohol (92 % ee) at
roughly the same rate [47] , while Gene Ãt's modification of the original procedure,
preparing the catalyst in situ and employing a hydrogen pressure of one atmos-
phere, allows the reaction to be performed without special apparatus*. Note that
other ligands have been employed for the ruthenium catalysed reduction of b-
ketoesters. For example, a new diphosphine (BisP*), ()-ephedrine and other
amino alcohols (for asymmetric transfer hydrogenation of arylketones and b-
ketoesters*) are described later, in the relevant experimental section.
Reduction of diketones such as pentane-2,4-dione using (R)-BiNAP±RuCl 2
under hydrogen (75±100 atm) gives the corresponding diol, in this case (R),(R)-
2,4-pentanediol with an excellent diastereomer ratio (98 %) and optical purity
(>99 %) [48] .
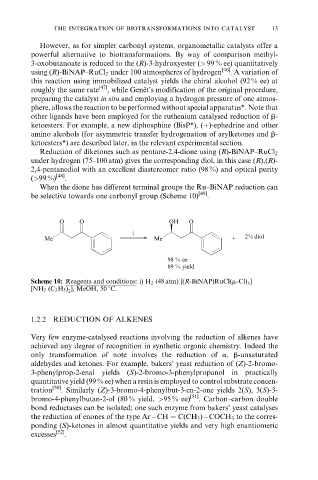
When the dione has different terminal groups the Ru±BiNAP reduction can
be selective towards one carbonyl group (Scheme 10) [49] .
O O OH O
i
Me Me + 2% diol
98 % ee
89 % yield
Scheme 10: Reagents and conditions: i) H 2 (48 atm) [(R-BiNAP)RuCl(m-Cl) ]
3
[NH 2 (C 2 H 5 ) ], MeOH, 50 8C.
2
1.2.2 REDUCTION OF ALKENES
Very few enzyme-catalysed reactions involving the reduction of alkenes have
achieved any degree of recognition in synthetic organic chemistry. Indeed the
only transformation of note involves the reduction of a, b-unsaturated
aldehydes and ketones. For example, bakers' yeast reduction of (Z)-2-bromo-
3-phenylprop-2-enal yields (S)-2-bromo-3-phenylpropanol in practically
quantitative yield (99 % ee) when a resin is employed to control substrate concen-
tration [50] . Similarly (Z)-3-bromo-4-phenylbut-3-en-2-one yields 2(S), 3(S)-3-
bromo-4-phenylbutan-2-ol (80 % yield, >95 % ee) [51] . Carbon±carbon double
bond reductases can be isolated; one such enzyme from bakers' yeast catalyses
the reduction of enones of the type ArÿCH C(CH 3 )ÿCOCH 3 to the corres-
ponding (S)-ketones in almost quantitative yields and very high enantiomeric
excesses [52] .