Page 33 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 33
16 hydrolysis, oxidation and reduction
dehydroamino acids. One of the more popular ligands for general usage has
been Burk's DuPHOS (Scheme 13)* [63] .
Me Me
P
CO 2 Me
i (CH 3 CH 2 ) 2 CH CO 2 Me
NHCOMe MeCONH H
P
Me Me
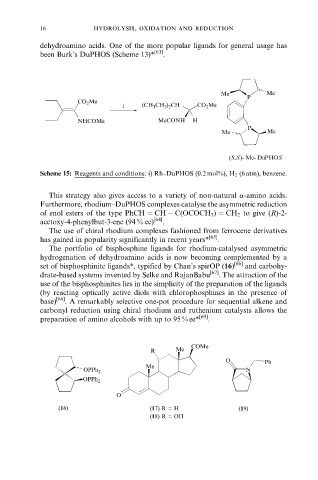
(S,S )- Me- DuPHOS
Scheme 15: Reagents and conditions: i) Rh±DuPHOS (0.2 mol%), H 2 (6 atm), benzene.
This strategy also gives access to a variety of non-natural a-amino acids.
Furthermore, rhodium±DuPHOS complexes catalyse the asymmetric reduction
of enol esters of the type PhCH CH ÿ C(OCOCH 3 ) CH 2 to give (R)-2-
acetoxy-4-phenylbut-3-ene (94 % ee) [64] .
The use of chiral rhodium complexes fashioned from ferrocene derivatives
has gained in popularity significantly in recent years* [65] .
The portfolio of bisphosphine ligands for rhodium-catalysed asymmetric
hydrogenation of dehydroamino acids is now becoming complemented by a
set of bisphosphinite ligands*, typified by Chan's spirOP (16) [66] and carbohy-
drate-based systems invented by Selke and RajanBabu [67] . The attraction of the
use of the bisphosphinites lies in the simplicity of the preparation of the ligands
(by reacting optically active diols with chlorophosphines in the presence of
base) [68] . A remarkably selective one-pot procedure for sequential alkene and
carbonyl reduction using chiral rhodium and ruthenium catalysts allows the
preparation of amino alcohols with up to 95 % ee* [69] .
COMe
R Me
O Ph
Me
OPPh 2 N
OPPh 2
O
(16) (17) R = H (19)
(18) R = OH