Page 45 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 45
28 hydrolysis, oxidation and reduction
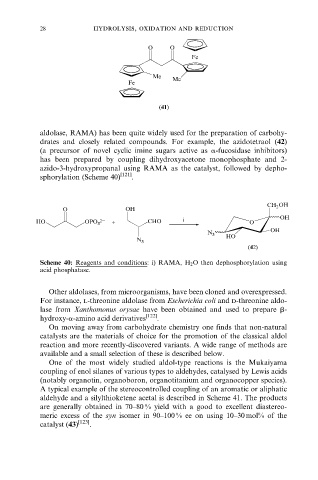
O O
Fe
Me
Fe Me
(41)
aldolase, RAMA) has been quite widely used for the preparation of carbohy-
drates and closely related compounds. For example, the azidotetraol (42)
(a precursor of novel cyclic imine sugars active as a-fucosidase inhibitors)
has been prepared by coupling dihydroxyacetone monophosphate and 2-
azido-3-hydroxypropanal using RAMA as the catalyst, followed by depho-
sphorylation (Scheme 40) [121] .
CH 2 OH
O OH
2− i OH
HO OPO 3 + CHO O
OH
N 3
HO
N 3
(42)
Scheme 40: Reagents and conditions: i) RAMA, H 2 O then dephosphorylation using
acid phosphatase.
Other aldolases, from microorganisms, have been cloned and overexpressed.
For instance, l-threonine aldolase from Escherichia coli and d-threonine aldo-
lase from Xanthomonus orysae have been obtained and used to prepare b-
hydroxy-a-amino acid derivatives [122] .
On moving away from carbohydrate chemistry one finds that non-natural
catalysts are the materials of choice for the promotion of the classical aldol
reaction and more recently-discovered variants. A wide range of methods are
available and a small selection of these is described below.
One of the most widely studied aldol-type reactions is the Mukaiyama
coupling of enol silanes of various types to aldehydes, catalysed by Lewis acids
(notably organotin, organoboron, organotitanium and organocopper species).
A typical example of the stereocontrolled coupling of an aromatic or aliphatic
aldehyde and a silylthioketene acetal is described in Scheme 41. The products
are generally obtained in 70±80 % yield with a good to excellent diastereo-
meric excess of the syn isomer in 90±100 % ee on using 10±30 mol% of the
catalyst (43) [123] .