Page 49 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 49
32 hydrolysis, oxidation and reduction
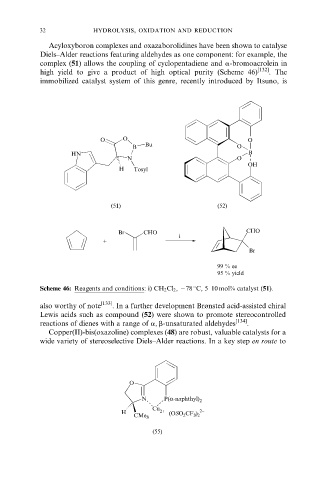
Acyloxyboron complexes and oxazaborolidines have been shown to catalyse
Diels±Alder reactions featuring aldehydes as one component: for example, the
complex (51) allows the coupling of cyclopentadiene and a-bromoacrolein in
high yield to give a product of high optical purity (Scheme 46) [132] . The
immobilized catalyst system of this genre, recently introduced by Itsuno, is
O O O
Bu
B O
HN B
N O
OH
H Tosyl
(51) (52)
Br CHO CHO
i
+
Br
99 % ee
95 % yield
Scheme 46: Reagents and conditions: i) CH 2 Cl 2 , ÿ78 C, 5±10 mol% catalyst (51).
also worthy of note [133] . In a further development Brùnsted acid-assisted chiral
Lewis acids such as compound (52) were shown to promote stereocontrolled
reactions of dienes with a range of a, b-unsaturated aldehydes [134] .
Copper(II)-bis(oxazoline) complexes (48) are robust, valuable catalysts for a
wide variety of stereoselective Diels±Alder reactions. In a key step en route to
O
N P(α-naphthyl) 2
Cu
H 2+ (OSO 2 CF 3 ) 2 2−
CMe 3
(55)