Page 240 - Chalcogenide Glasses for Infrared Optics
P. 240
216 Cha pte r Ni ne
by the U.S. Army covering the period from 1982 to 1985. The author
was fortunate in recruiting his good friend and former colleague at
TI to join AMI and take over all the crystalline materials work. George
Cronin had been a leader in developing methods to produce many
crystalline materials including all the III–V materials since joining
TI in 1958. Of particular interest to AMI was his work with GaAs and
high-purity vacuum float zoned silicon; later we discuss the AMI
activity in producing these two materials.
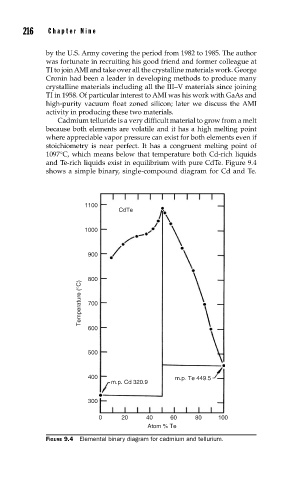
Cadmium telluride is a very difficult material to grow from a melt
because both elements are volatile and it has a high melting point
where appreciable vapor pressure can exist for both elements even if
stoichiometry is near perfect. It has a congruent melting point of
1097°C, which means below that temperature both Cd-rich liquids
and Te-rich liquids exist in equilibrium with pure CdTe. Figure 9.4
shows a simple binary, single-compound diagram for Cd and Te.
1100
CdTe
1000
900
800
Temperature (°C) 700
600
500
400 m.p. Te 449.5
m.p. Cd 320.9
300
0 20 40 60 80 100
Atom % Te
FIGURE 9.4 Elemental binary diagram for cadmium and tellurium.