Page 238 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 238
216 CORROSION CONTROL AND PREVENTION
4.4 CORROSION INHIBITORS
A corrosion inhibitor in general terms is a chemical substance that when added in a
small amount to an environment effectively reduces the corrosion rate of a metal or
alloy exposed to the corrosive environment. A more precise definition of an inhibitor
is not possible because of the number of mechanistic and/or chemical considerations
when classifying corrosion inhibitors.
In most cases, corrosion inhibition is achieved through interaction or reaction
between the corrosion inhibitor and the metal surface, resulting in the formation of
an inhibitive surface film. In some cases, the chemistry of the environment may be
modified to make it less corrosive, such as adjusting the pH of the solution to pro-
mote passivation, scavenging dissolved oxygen or neutralizing acidic species. Anodic
inhibitors such as chromates, molybdates, tungstates, phosphates, and nitrites func-
tion by interfering with the corrosion reaction occurring at the anodic site. Carbonates
and arsenates inhibit the current flow by interfering with the cathodic reaction. Film
forming inhibitors such as organic amines and imidazolines function as anodic or
cathodic or both anodic and cathodic inhibitors.
Corrosion inhibition is used internally with carbon steel pipes and vessels as an
economic corrosion control alternative to stainless steels and alloys, coatings, or non-
metallic composites. A particular advantage of corrosion inhibition is that it can be
implemented or changed in situ without disruption of the process. For example, in
processes that produce environments of increasing corrosivity with time, such as
“souring” oil fields, corrosion can be effectively controlled with a suitable inhibitor.
The major industries that use corrosion inhibitors are: petroleum production and
refining, chemical and heavy industrial manufacturing, and the product additive
industry. The usage summary of corrosion inhibitors in various industries is given in
Table 4.9.
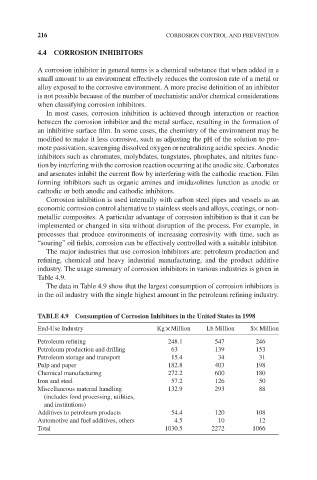
The data in Table 4.9 show that the largest consumption of corrosion inhibitors is
in the oil industry with the single highest amount in the petroleum refining industry.
TABLE 4.9 Consumption of Corrosion Inhibitors in the United States in 1998
End-Use Industry Kg × Million Lb Million $× Million
Petroleum refining 248.1 547 246
Petroleum production and drilling 63 139 153
Petroleum storage and transport 15.4 34 31
Pulp and paper 182.8 403 198
Chemical manufacturing 272.2 600 180
Iron and steel 57.2 126 50
Miscellaneous material handling 132.9 293 88
(includes food processing, utilities,
and institutions)
Additives to petroleum products 54.4 120 108
Automotive and fuel additives, others 4.5 10 12
Total 1030.5 2272 1066