Page 127 - Chemical process engineering design and economics
P. 127
Process Circuit Analysis 111
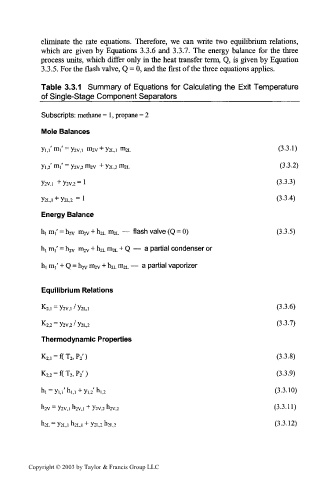
eliminate the rate equations. Therefore, we can write two equilibrium relations,
which are given by Equations 3.3.6 and 3.3.7. The energy balance for the three
process units, which differ only in the heat transfer term, Q, is given by Equation
3.3.5. For the flash valve, Q = 0, and the first of the three equations applies.
Table 3.3.1 Summary of Equations for Calculating the Exit Temperature
of Single-Stage Component Separators___________________
Subscripts: methane = 1, propane = 2
Mole Balances
m
Yi.i' i' = Y2v,i m 2V + y 2L,i rn 2L (3.3.1)
yi,2 m,' = y 2V>2 m 2v + y 2L, 2 m 2L (3.3.2)
V2V.1 + V2V.2 = 1 (3-3.3)
Y2L.1 + V2L.2 = 1 (3-3.4)
Energy Balance
— flash valve (Q = 0) (3.3.5)
hj m,' = h 2V m 2V + h 2L m 2L
m 2V + h 2L m 2L + Q — a partial condenser or
h, m,' = h 2V
hi m,' + Q = h 2V m 2V + h 2L m 2L — a partial vaporizer
Equilibrium Relations
K 2,i = y 2V,i / y 2L,i (3-3-6)
(3-3.7)
K 2, 2 = y 2v,2 / y 2L, 2
Thermodynamic Properties
K 2,, = f(T 2 ,P 2 ') (3.3.8)
K 2;2 = f(T 2 ,P 2 ') (3.3.9)
hi = yu'h,,, + y,, 2'h, ]2 (3.3.10)
h 2v = y 2v,i h 2V>] + y 2v, 2 h 2V>2 (3.3.11)
h 2L = y 2L,i Vi + Y2L.2 Vz (3.3.12)
Copyright © 2003 by Taylor & Francis Group LLC