Page 126 - Chemical process engineering design and economics
P. 126
110 Chapter 3
r^\ te
Flash Valve
Partial Condenser Partial Vaporizer
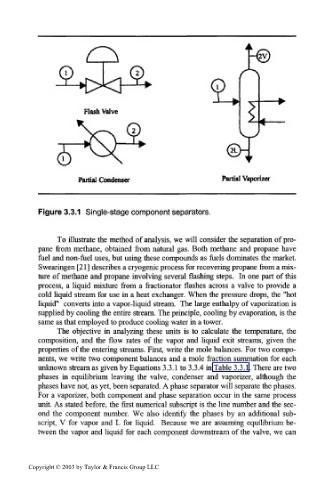
Figure 3.3.1 Single-stage component separators.
To illustrate the method of analysis, we will consider the separation of pro-
pane from methane, obtained from natural gas. Both methane and propane have
fuel and non-fuel uses, but using these compounds as fuels dominates the market.
Swearingen [21] describes a cryogenic process for recovering propane from a mix-
ture of methane and propane involving several flashing steps. In one part of this
process, a liquid mixture from a fractionator flashes across a valve to provide a
cold liquid stream for use in a heat exchanger. When the pressure drops, the "hot
liquid" converts into a vapor-liquid stream. The large enthalpy of vaporization is
supplied by cooling the entire stream. The principle, cooling by evaporation, is the
same as that employed to produce cooling water in a tower.
The objective in analyzing these units is to calculate the temperature, the
composition, and the flow rates of the vapor and liquid exit streams, given the
properties of the entering streams. First, write the mole balances. For two compo-
nents, we write two component balances and a mole fraction summation for each
unknown stream as given by Equations 3.3.1 to 3.3.4 in Table 3.3.1. There are two
phases in equilibrium leaving the valve, condenser and vaporizer, although the
phases have not, as yet, been separated. A phase separator will separate the phases.
For a vaporizer, both component and phase separation occur in the same process
unit. As stated before, the first numerical subscript is the line number and the sec-
ond the component number. We also identify the phases by an additional sub-
script, V for vapor and L for liquid. Because we are assuming equilibrium be-
tween the vapor and liquid for each component downstream of the valve, we can
Copyright © 2003 by Taylor & Francis Group LLC