Page 209 - Chiral Separation Techniques
P. 209
7.2 Different Approaches for Derivatization Chromatography 187
Table 7-1. Different approaches for derivatization chromatography
Type Educt Derivatizing Agent Stationary type of binding
number type phase or reaction
I one one unichiral achiral covalent
II multiple one achiral or achiral or covalent
unichiral unichiral
III one one achiral unichiral covalent
IV one multiple achiral unichiral covalent
V one one achiral achiral enzymatic
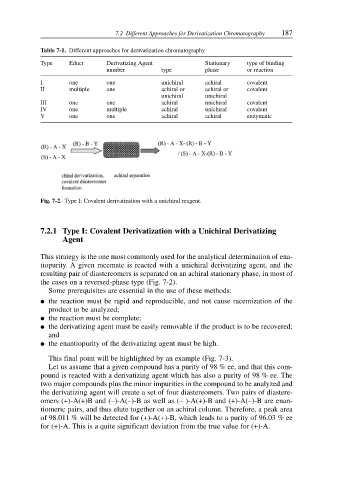
Fig. 7-2. Type I: Covalent derivatization with a unichiral reagent.
7.2.1 Type I: Covalent Derivatization with a Unichiral Derivatizing
Agent
This strategy is the one most commonly used for the analytical determination of ena-
tiopurity. A given racemate is reacted with a unichiral derivatizing agent, and the
resulting pair of diastereomers is separated on an achiral stationary phase, in most of
the cases on a reversed-phase type (Fig. 7-2).
Some prerequisites are essential in the use of these methods:
the reaction must be rapid and reproducible, and not cause racemization of the
product to be analyzed;
the reaction must be complete;
the derivatizing agent must be easily removable if the product is to be recovered;
and
the enantiopurity of the derivatizing agent must be high.
This final point will be highlighted by an example (Fig. 7-3).
Let us assume that a given compound has a purity of 98 % ee, and that this com-
pound is reacted with a derivatizing agent which has also a purity of 98 % ee. The
two major compounds plus the minor impurities in the compound to be analyzed and
the derivatizing agent will create a set of four diastereomers. Two pairs of diastere-
omers (+)-A(+)B and (–)-A(–)-B as well as (– )-A(+)-B and (+)-A(–)-B are enan-
tiomeric pairs, and thus elute together on an achiral column. Therefore, a peak area
of 98.011 % will be detected for (+)-A(+)-B, which leads to a purity of 96.03 % ee
for (+)-A. This is a quite significant deviation from the true value for (+)-A.