Page 210 - Chiral Separation Techniques
P. 210
188 7 Chiral Derivatization Chromatography
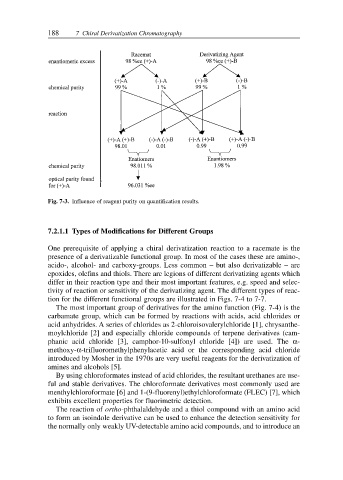
Fig. 7-3. Influence of reagent purity on quantification results.
7.2.1.1 Types of Modifications for Different Groups
One prerequisite of applying a chiral derivatization reaction to a racemate is the
presence of a derivatizable functional group. In most of the cases these are amino-,
acido-, alcohol- and carboxy-groups. Less common – but also derivatizable – are
epoxides, olefins and thiols. There are legions of different derivatizing agents which
differ in their reaction type and their most important features, e.g. speed and selec-
tivity of reaction or sensitivity of the derivatizing agent. The different types of reac-
tion for the different functional groups are illustrated in Figs. 7-4 to 7-7.
The most important group of derivatives for the amino function (Fig. 7-4) is the
carbamate group, which can be formed by reactions with acids, acid chlorides or
acid anhydrides. A series of chlorides as 2-chloroisovalerylchloride [1], chrysanthe-
moylchloride [2] and especially chloride compounds of terpene derivatives (cam-
phanic acid chloride [3], camphor-10-sulfonyl chloride [4]) are used. The α-
methoxy-α-trifluoromethylphenylacetic acid or the corresponding acid chloride
introduced by Mosher in the 1970s are very useful reagents for the derivatization of
amines and alcohols [5].
By using chloroformates instead of acid chlorides, the resultant urethanes are use-
ful and stable derivatives. The chloroformate derivatives most commonly used are
menthylchloroformate [6] and 1-(9-fluorenyl)ethylchloroformate (FLEC) [7], which
exhibits excellent properties for fluorimetric detection.
The reaction of ortho-phthalaldehyde and a thiol compound with an amino acid
to form an isoindole derivative can be used to enhance the detection sensitivity for
the normally only weakly UV-detectable amino acid compounds, and to introduce an