Page 212 - Chiral Separation Techniques
P. 212
190 7 Chiral Derivatization Chromatography
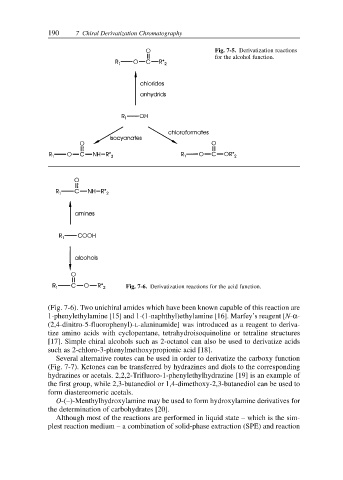
Fig. 7-5. Derivatization reactions
for the alcohol function.
Fig. 7-6. Derivatization reactions for the acid function.
(Fig. 7-6). Two unichiral amides which have been known capable of this reaction are
1-phenylethylamine [15] and 1-(1-naphthyl)ethylamine [16]. Marfey’s reagent [N-α-
(2,4-dinitro-5-fluorophenyl)-L-alaninamide] was introduced as a reagent to deriva-
tize amino acids with cyclopentane, tetrahydroisoquinoline or tetraline structures
[17]. Simple chiral alcohols such as 2-octanol can also be used to derivatize acids
such as 2-chloro-3-phenylmethoxypropionic acid [18].
Several alternative routes can be used in order to derivatize the carboxy function
(Fig. 7-7). Ketones can be transferred by hydrazines and diols to the corresponding
hydrazines or acetals. 2,2,2-Trifluoro-1-phenylethylhydrazine [19] is an example of
the first group, while 2,3-butanediol or 1,4-dimethoxy-2,3-butanediol can be used to
form diastereomeric acetals.
O-(–)-Menthylhydroxylamine may be used to form hydroxylamine derivatives for
the determination of carbohydrates [20].
Although most of the reactions are performed in liquid state – which is the sim-
plest reaction medium – a combination of solid-phase extraction (SPE) and reaction