Page 214 - Chiral Separation Techniques
P. 214
192 7 Chiral Derivatization Chromatography
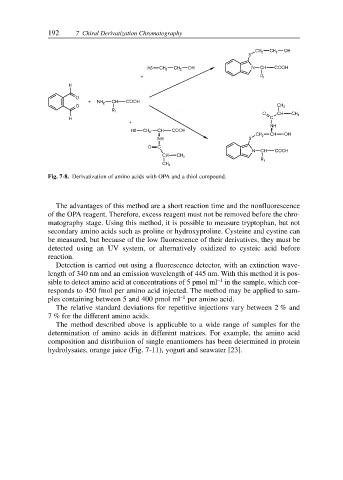
Fig. 7-8. Derivatization of amino acids with OPA and a thiol compound.
The advantages of this method are a short reaction time and the nonfluorescence
of the OPA reagent. Therefore, excess reagent must not be removed before the chro-
matography stage. Using this method, it is possible to measure tryptophan, but not
secondary amino acids such as proline or hydroxyproline. Cysteine and cystine can
be measured, but because of the low fluorescence of their derivatives, they must be
detected using an UV system, or alternatively oxidized to cysteic acid before
reaction.
Detection is carried out using a fluorescence detector, with an extinction wave-
length of 340 nm and an emission wavelength of 445 nm. With this method it is pos-
–1
sible to detect amino acid at concentrations of 5 pmol ml in the sample, which cor-
responds to 450 fmol per amino acid injected. The method may be applied to sam-
–1
ples containing between 5 and 400 pmol ml per amino acid.
The relative standard deviations for repetitive injections vary between 2 % and
7 % for the different amino acids.
The method described above is applicable to a wide range of samples for the
determination of amino acids in different matrices. For example, the amino acid
composition and distribution of single enantiomers has been determined in protein
hydrolysates, orange juice (Fig. 7-11), yogurt and seawater [23].