Page 213 - Chiral Separation Techniques
P. 213
7.2 Different Approaches for Derivatization Chromatography 191
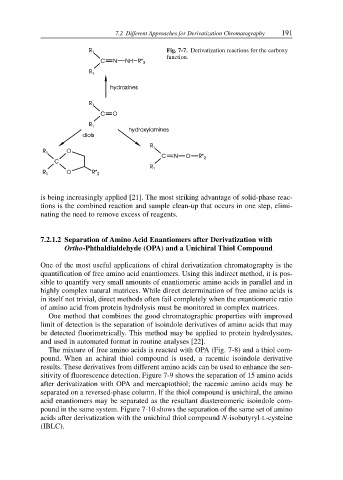
Fig. 7-7. Derivatization reactions for the carboxy
function.
is being increasingly applied [21]. The most striking advantage of solid-phase reac-
tions is the combined reaction and sample clean-up that occurs in one step, elimi-
nating the need to remove excess of reagents.
7.2.1.2 Separation of Amino Acid Enantiomers after Derivatization with
Ortho-Phthaldialdehyde (OPA) and a Unichiral Thiol Compound
One of the most useful applications of chiral derivatization chromatography is the
quantification of free amino acid enantiomers. Using this indirect method, it is pos-
sible to quantify very small amounts of enantiomeric amino acids in parallel and in
highly complex natural matrices. While direct determination of free amino acids is
in itself not trivial, direct methods often fail completely when the enantiomeric ratio
of amino acid from protein hydrolysis must be monitored in complex matrices.
One method that combines the good chromatographic properties with improved
limit of detection is the separation of isoindole derivatives of amino acids that may
be detected fluorimetrically. This method may be applied to protein hydrolysates,
and used in automated format in routine analyses [22].
The mixture of free amino acids is reacted with OPA (Fig. 7-8) and a thiol com-
pound. When an achiral thiol compound is used, a racemic isoindole derivative
results. These derivatives from different amino acids can be used to enhance the sen-
sitivity of fluorescence detection. Figure 7-9 shows the separation of 15 amino acids
after derivatization with OPA and mercaptothiol; the racemic amino acids may be
separated on a reversed-phase column. If the thiol compound is unichiral, the amino
acid enantiomers may be separated as the resultant diastereomeric isoindole com-
pound in the same system. Figure 7-10 shows the separation of the same set of amino
acids after derivatization with the unichiral thiol compound N-isobutyryl-L-cysteine
(IBLC).