Page 211 - Chiral Separation Techniques
P. 211
7.2 Different Approaches for Derivatization Chromatography 189
unichiral center which renders the amino acid derivatives separable on a reversed-
phase column. An example of this technique will be given later.
A series of reactions was developed to transfer amines to ureido- and thioureido-
derivatives for separation. The reaction of ureido-derivatives is widely used by the
reaction with 1-phenylethyl isocyanate (PEIC) [8] or the naphthyl-analogue 1-(1-
naphthyl)ethyl isocyanate (NEIC) [9]. Both reactions can be used not only for chiral
amines but also for alcohols and thiols.
Thioisocyanates as derivatizing reagents are often based on unichiral carbohy-
drate compounds. One very frequently used reagent in the analysis of amino acids is
2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl isothiocyanate (TAGIT or GITC) [10].
Other derivatizing reagents of the same type are based on galactose or arabinose as
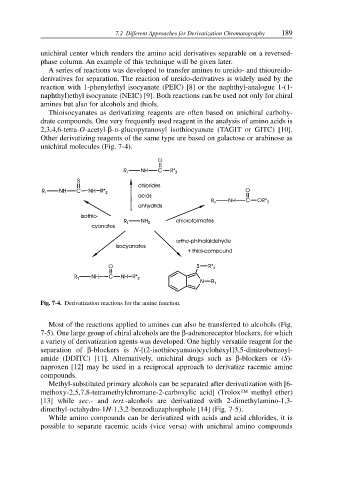
unichiral molecules (Fig. 7-4).
Fig. 7-4. Derivatization reactions for the amine function.
Most of the reactions applied to amines can also be transferred to alcohols (Fig.
7-5). One large group of chiral alcohols are the β-adrenoreceptor blockers, for which
a variety of derivatization agents was developed. One highly versatile reagent for the
separation of β-blockers is N-[(2-isothiocyanato)cyclohexyl]3,5-dinitrobenzoyl-
amide (DDITC) [11]. Alternatively, unichiral drugs such as β-blockers or (S)-
naproxen [12] may be used in a reciprocal approach to derivatize racemic amine
compounds.
Methyl-substituted primary alcohols can be separated after derivatization with [6-
methoxy-2,5,7,8-tetramethylchromane-2-carboxylic acid] (Trolox™ methyl ether)
[13] while sec.- and tert.-alcohols are derivatized with 2-dimethylamino-1,3-
dimethyl-octahydro-1H-1,3,2-benzodiazaphosphole [14] (Fig. 7-5).
While amino compounds can be derivatized with acids and acid chlorides, it is
possible to separate racemic acids (vice versa) with unichiral amino compounds