Page 12 - Color Atlas of Biochemistry
P. 12
Chemistry 3
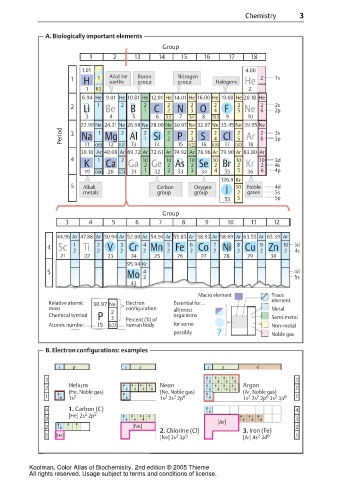
A. Biologically important elements
Group
1 2 13 14 15 16 17 18
1.01 4.00
1 H 1 Alkaline Boron Nitrogen Halogens He 2 1s
group
earths
group
1 63 2
6.94 He 9.01 He 10.81 He 12.01 He 14.01 He 16.00 He 19.00 He 20.18 He
? Be ? 1 C 2 N 3 O 4 F 5 Ne 6 2p
Li
B
2 1 2 2 2 2 2 2 2 2s
3 4 5 6 9.5 7 1.4 8 25.5 9 10
22.99 Ne 24.31 Ne 26.98 Ne 28.09 Ne 30.97 Ne 32.07 Ne 35.45 Ne 39.95 Ne
Period 3 Na 1 Mg 2 Al 2 2 2 2 2 2 3s
?
5 Ar
2 P
1 Si
3 S
4 Cl
6
3p
0.22 16
15
11
17
0.01 13
12
14
18
0.03
0.03
0.05
39.10 Ar 40.08 Ar 69.72 Ar 72.61 Ar 74.92 Ar 78.96 Ar 79.90 Ar 83.80 Ar
4 1 2 10 10 10 10 10 10 3d
?
?
K Ca Ga 2 Ge 2 As 2 Se 2 Br 2 Kr 2 4s
19 0.06 20 0.31 31 1 32 2 33 3 34 4 35 5 36 6 4p
126.9 Kr
5 Alkali Carbon Oxygen 10 Noble 4d
metals group group I 2 gases 5s
53 5 5p
Group
3 4 5 6 7 8 9 10 11 12
44.96 Ar 47.88 Ar 50.94 Ar 52.00 Ar 54.94 Ar 55.85 Ar 58.93 Ar 58.69 Ar 63.55 Ar 65.39 Ar
4 Sc 1 Ti 2 V 3 Cr 4 Mn 5 Fe 6 Co 7 Ni 8 Cu 9 Zn 10 3d
2
2
2
4s
2
2
2
2
2
2
2
21 22 23 24 25 26 27 28 29 30
95.94 Kr
5 4 4d
Mo 2 5s
42
Macro element Trace
element
Relative atomic 30.97 Ne Electron Essential for...
mass configuration all/most Metal
P 3 Percent (%) of Semi-metal
Chemical symbol 2 organisms
Atomic number 15 0.22 human body for some Non-metal
possibly ? Noble gas
B. Electron configurations: examples
s p s p s p d
3 3
Helium Neon Argon
2 2
(He, Noble gas) (Ne, Noble gas) (Ar, Noble gas)
1 1s 2 1s 2s 2p 6 1s 2s 2p 3s 3p 6 1
2
2
2
2
2
6
4 1. Carbon (C) 4
2
[He] 2s 2p 2
3 3
[Ar]
2 [Ne] 2
2. Chlorine (Cl) 3. Iron (Fe)
1 [He] 2 5 2 6 1
[Ne] 3s 3p [Ar] 4s 3d
Koolman, Color Atlas of Biochemistry, 2nd edition © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.