Page 115 - Computational Modeling in Biomedical Engineering and Medical Physics
P. 115
104 Computational Modeling in Biomedical Engineering and Medical Physics
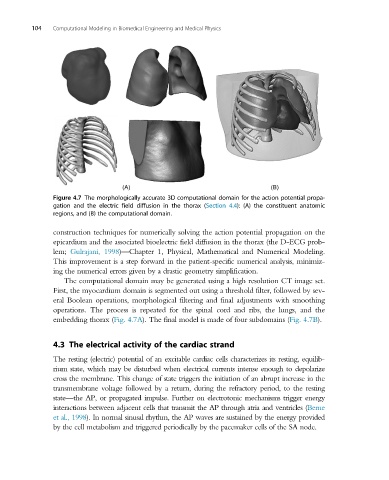
Figure 4.7 The morphologically accurate 3D computational domain for the action potential propa-
gation and the electric field diffusion in the thorax (Section 4.4): (A) the constituent anatomic
regions, and (B) the computational domain.
construction techniques for numerically solving the action potential propagation on the
epicardium and the associated bioelectric field diffusion in the thorax (the D-ECG prob-
lem; Gulrajani, 1998)—Chapter 1, Physical, Mathematical and Numerical Modeling.
This improvement is a step forward in the patient-specific numerical analysis, minimiz-
ing the numerical errors given by a drastic geometry simplification.
The computational domain may be generated using a high resolution CT image set.
First, the myocardium domain is segmented out using a threshold filter, followed by sev-
eral Boolean operations, morphological filtering and final adjustments with smoothing
operations. The process is repeated for the spinal cord and ribs, the lungs, and the
embedding thorax (Fig. 4.7A). The final model is made of four subdomains (Fig. 4.7B).
4.3 The electrical activity of the cardiac strand
The resting (electric) potential of an excitable cardiac cells characterizes its resting, equilib-
rium state, which may be disturbed when electrical currents intense enough to depolarize
cross the membrane. This change of state triggers the initiation of an abrupt increase in the
transmembrane voltage followed by a return, during the refractory period, to the resting
state—the AP, or propagated impulse. Further on electrotonic mechanisms trigger energy
interactions between adjacent cells that transmit the AP through atria and ventricles (Berne
et al., 1998). In normal sinusal rhythm, the AP waves are sustained by the energy provided
by the cell metabolism and triggered periodically by the pacemaker cells of the SA node.