Page 130 - Corrosion Engineering Principles and Practice
P. 130
104 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 105
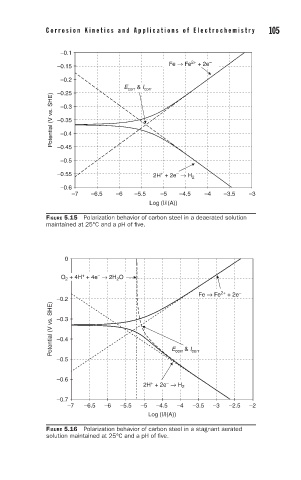
–0.1
–
2+
–0.15 Fe → Fe + 2e
–0.2
E corr & I corr
–0.25
Potential (V vs. SHE) –0.35
–0.3
–0.4
–0.45
–0.5
–0.55 2H + 2e → H 2
–
+
–0.6
–7 –6.5 –6 –5.5 –5 –4.5 –4 –3.5 –3
Log (|i|(A))
FIGURE 5.15 Polarization behavior of carbon steel in a deaerated solution
maintained at 25°C and a pH of five.
0
+
–
O + 4H + 4e → 2H O
2
2
2+
–
Fe → Fe + 2e
–0.2
Potential (V vs. SHE) –0.3
–0.4
–0.5 E corr & I corr
–0.6
–
+
2H + 2e → H 2
–0.7
–7 –6.5 –6 –5.5 –5 –4.5 –4 –3.5 –3 –2.5 –2
Log (|i|(A))
FIGURE 5.16 Polarization behavior of carbon steel in a stagnant aerated
solution maintained at 25°C and a pH of five.