Page 135 - Corrosion Engineering Principles and Practice
P. 135
110 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 111
A small amount of chloride ion (1 percent) was also present in this
acidic chemical.
In this example, the potential scan rate was 0.5 mV s and the
−1
−2
scan direction was reversed at 0.1 mA cm . Coupon immersion tests
were run in the same environment for 840 hours. The S31600 steel
specimens were exposed to the liquid, at the vapor/liquid interface,
and in the vapor. The reason for the three exposures was that in most
storage situations, the containment vessel would be exposed to a
vapor/liquid interface and a vapor phase at least part of the time.
Corrosion in these regions can be very different from liquid exposures.
The specimens were also fitted with artificial crevice formers.
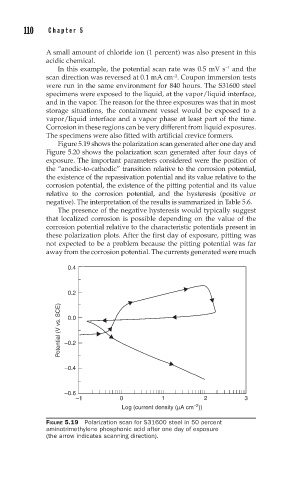
Figure 5.19 shows the polarization scan generated after one day and
Figure 5.20 shows the polarization scan generated after four days of
exposure. The important parameters considered were the position of
the “anodic-to-cathodic” transition relative to the corrosion potential,
the existence of the repassivation potential and its value relative to the
corrosion potential, the existence of the pitting potential and its value
relative to the corrosion potential, and the hysteresis (positive or
negative). The interpretation of the results is summarized in Table 5.6.
The presence of the negative hysteresis would typically suggest
that localized corrosion is possible depending on the value of the
corrosion potential relative to the characteristic potentials present in
these polarization plots. After the first day of exposure, pitting was
not expected to be a problem because the pitting potential was far
away from the corrosion potential. The currents generated were much
0.4
0.2
Potential (V vs. SCE) –0.2
0.0
–0.4
–0.6
–1 0 1 2 3
–2
Log (current density (µA cm ))
FIGURE 5.19 Polarization scan for S31600 steel in 50 percent
aminotrimethylene phosphonic acid after one day of exposure
(the arrow indicates scanning direction).