Page 131 - Corrosion Engineering Principles and Practice
P. 131
106 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 107
that is, pH of five at 25°C, with the exception that the environment is
aerated and stagnant. In this situation the reduction of oxygen shown
in Eq. (5.21) now becomes a possible second cathodic reaction.
−
+
O + 4H + 4e → 2H O (5.21)
2
2
In order to model the polarization plot, the total cathodic current
corresponding to the sum of the currents of the hydrogen reaction
and oxygen reduction has to be balanced by the single anodic current.
The intercept where the opposing currents are balanced occurs at an
E corr of −0.33 V vs. SHE and, since the surface area is still 1 cm , an i corr
2
−1
−2
of 8.2 µA cm or 0.1 mm y . It should be noted that the current for the
reduction of oxygen is constant across the potential range shown in
Fig. 5.16 and has the value of 6.3 µA cm .
−2
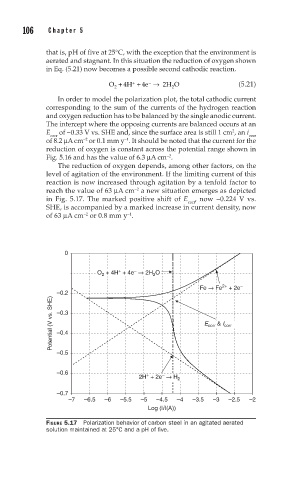
The reduction of oxygen depends, among other factors, on the
level of agitation of the environment. If the limiting current of this
reaction is now increased through agitation by a tenfold factor to
reach the value of 63 µA cm a new situation emerges as depicted
−2
in Fig. 5.17. The marked positive shift of E corr , now −0.224 V vs.
SHE, is accompanied by a marked increase in current density, now
of 63 µA cm or 0.8 mm y .
−2
−1
0
+
–
O + 4H + 4e → 2H O
2
2
–
2+
Fe → Fe + 2e
–0.2
Potential (V vs. SHE) –0.3 E corr & I corr
–0.4
–0.5
–0.6
–
+
2H + 2e → H 2
–0.7
–7 –6.5 –6 –5.5 –5 –4.5 –4 –3.5 –3 –2.5 –2
Log (|i|(A))
FIGURE 5.17 Polarization behavior of carbon steel in an agitated aerated
solution maintained at 25°C and a pH of five.