Page 242 - Corrosion Engineering Principles and Practice
P. 242
216 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 217
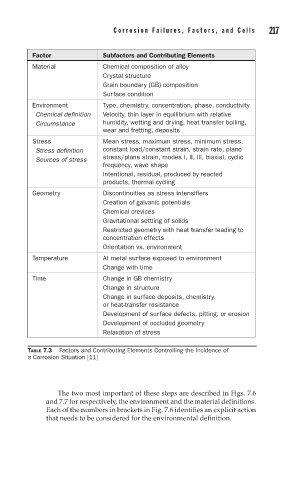
Factor Subfactors and Contributing Elements
Material Chemical composition of alloy
Crystal structure
Grain boundary (GB) composition
Surface condition
Environment Type, chemistry, concentration, phase, conductivity
Chemical definition Velocity, thin layer in equilibrium with relative
Circumstance humidity, wetting and drying, heat transfer boiling,
wear and fretting, deposits
Stress Mean stress, maximum stress, minimum stress,
Stress definition constant load/constant strain, strain rate, plane
Sources of stress stress/plane strain, modes I, II, III, biaxial, cyclic
frequency, wave shape
Intentional, residual, produced by reacted
products, thermal cycling
Geometry Discontinuities as stress intensifiers
Creation of galvanic potentials
Chemical crevices
Gravitational settling of solids
Restricted geometry with heat transfer leading to
concentration effects
Orientation vs. environment
Temperature At metal surface exposed to environment
Change with time
Time Change in GB chemistry
Change in structure
Change in surface deposits, chemistry,
or heat-transfer resistance
Development of surface defects, pitting, or erosion
Development of occluded geometry
Relaxation of stress
TABLE 7.3 Factors and Contributing Elements Controlling the Incidence of
a Corrosion Situation [11]
The two most important of these steps are described in Figs. 7.6
and 7.7 for respectively, the environment and the material definitions.
Each of the numbers in brackets in Fig. 7.6 identifies an explicit action
that needs to be considered for the environmental definition.