Page 251 - Corrosion Engineering Principles and Practice
P. 251
224 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 225
in the metal or the environment, from impressed currents, or from local
differences in the many factors described earlier that may cause
corrosion. Metal variations may be the result of composition differences.
A second phase constituent will have a different corrosion potential
compared to that of an adjacent solid solution*. The difference may be
in the thickness of a surface film at adjacent sites which in turn may
reflect metal differences in the substrate.
Any two or more of the cells described in the following sections
can operate at the same time. Sometimes they will work in synergy,
sometimes they will be opposed. For example, in a well casing, there
is usually an oxygen cell and a temperature cell, both tending to make
the deep pipe act as an anode. At the same time, there may be two or
more ordinary concentration cells, as the casing passes through
different strata of soil.
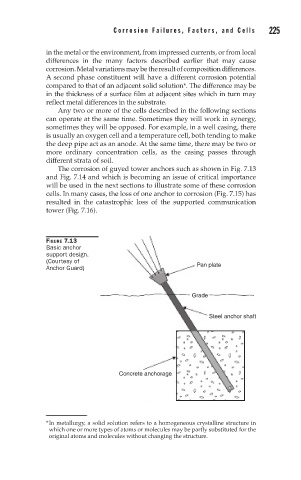
The corrosion of guyed tower anchors such as shown in Fig. 7.13
and Fig. 7.14 and which is becoming an issue of critical importance
will be used in the next sections to illustrate some of these corrosion
cells. In many cases, the loss of one anchor to corrosion (Fig. 7.15) has
resulted in the catastrophic loss of the supported communication
tower (Fig. 7.16).
FIGURE 7.13
Basic anchor
support design.
(Courtesy of
Anchor Guard) Pan plate
Grade
Steel anchor shaft
Concrete anchorage
* In metallurgy, a solid solution refers to a homogeneous crystalline structure in
which one or more types of atoms or molecules may be partly substituted for the
original atoms and molecules without changing the structure.