Page 250 - Corrosion Engineering Principles and Practice
P. 250
224 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 225
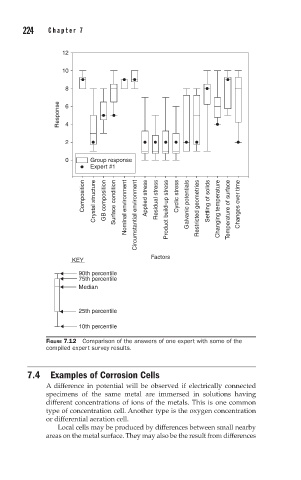
12
10
8
Response 6
4
2
0 Group response
Expert #1
Composition Crystal structure GB composition Surface condition Nominal environment Circumstantial environment Applied stress Residual stress Product build-up stress Cyclic stress Galvanic potentials Restricted geometries Settling of solids Changing temperature Temperature of surface Changes over time
KEY Factors
90th percentile
75th percentile
Median
25th percentile
10th percentile
FIGURE 7.12 Comparison of the answers of one expert with some of the
compiled expert survey results.
7.4 Examples of Corrosion Cells
A difference in potential will be observed if electrically connected
specimens of the same metal are immersed in solutions having
different concentrations of ions of the metals. This is one common
type of concentration cell. Another type is the oxygen concentration
or differential aeration cell.
Local cells may be produced by differences between small nearby
areas on the metal surface. They may also be the result from differences