Page 618 - Corrosion Engineering Principles and Practice
P. 618
580 C h a p t e r 1 3 C a t h o d i c P r o t e c t i o n 581
Γ must be continuous but all sections of one surface type do not have
to be contiguous. An ICCP system consists of the surfaces to be
protected, the anodes, the reference cells, and the power supply.
Anodes are defined by maintaining the potential at a constant value,
Φ : in Eq. (13.5):
A
Φ ( , ) = Φ A (13.5)
x
y
defining the current density as a constant, q , on a surface in
A
Eq. (13.6)
∂Φ( , ) y
x
∂ ( , ) y = q A (13.6)
n x
where Φ(x, y) is the electrical potential at the point (x, y)
n(x, y) is the normal to the surface at the point (x, y).
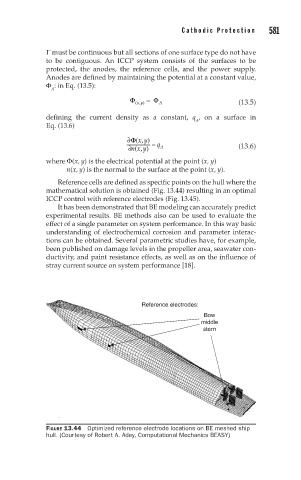
Reference cells are defined as specific points on the hull where the
mathematical solution is obtained (Fig. 13.44) resulting in an optimal
ICCP control with reference electrodes (Fig. 13.45).
It has been demonstrated that BE modeling can accurately predict
experimental results. BE methods also can be used to evaluate the
effect of a single parameter on system performance. In this way basic
understanding of electrochemical corrosion and parameter interac-
tions can be obtained. Several parametric studies have, for example,
been published on damage levels in the propeller area, seawater con-
ductivity, and paint resistance effects, as well as on the influence of
stray current source on system performance [18].
Reference electrodes:
Bow
middle
stern
FIGURE 13.44 Optimized reference electrode locations on BE meshed ship
hull. (Courtesy of Robert A. Adey, Computational Mechanics BEASY)