Page 718 - Corrosion Engineering Principles and Practice
P. 718
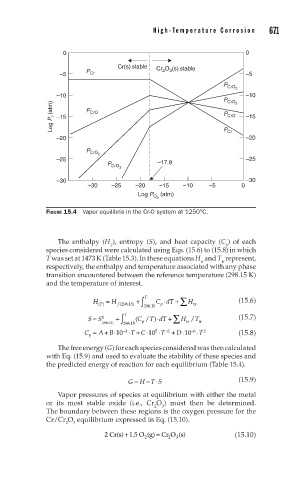
670 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 671
0 0
Cr(s) stable O (s) stable
–5 P Cr Cr 2 3 –5
P CrO 3
–10 –10
P CrO 2
Log P i (atm) –15 P CrO P CrO –15
–20 P Cr –20
P CrO
2
–25 –25
P CrO 3 –17.9
–30 –30
–30 –25 –20 –15 –10 –5 0
Log P (atm)
O
2
FIGURE 15.4 Vapor equilibria in the Cr-O system at 1250°C.
The enthalpy (H ), entropy (S), and heat capacity (C ) of each
p
T
species considered were calculated using Eqs. (15.6) to (15.8) in which
T was set at 1473 K (Table 15.3). In these equations H and T represent,
tr
tr
respectively, the enthalpy and temperature associated with any phase
transition encountered between the reference temperature (298.15 K)
and the temperature of interest.
H = H .15 ∫ T C dT + ∑ H (15.6)
⋅
+
f (298
)
T ( )
298 .15 p tr
S S 0 + ∫ T C ( / T dT + ∑ H / T (15.7)
⋅ )
=
(298 .15 ) 298 .15 p tr tr
+
+
C = A B⋅10 −3 ⋅ T C⋅10 5 T ⋅ −2 + D⋅10 −6 T ⋅ 2 (15.8)
p
The free energy (G) for each species considered was then calculated
with Eq. (15.9) and used to evaluate the stability of these species and
the predicted energy of reaction for each equilibrium (Table 15.4).
G = H T S (15.9)
⋅
−
Vapor pressures of species at equilibrium with either the metal
or its most stable oxide (i.e., Cr O ) must then be determined.
3
2
The boundary between these regions is the oxygen pressure for the
Cr/Cr O equilibrium expressed in Eq. (15.10).
2 3
=
+
2 Cr(s) 1.5 O (g) Cr O (s) (15.10)
2
3
2