Page 719 - Corrosion Engineering Principles and Practice
P. 719
672 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 673
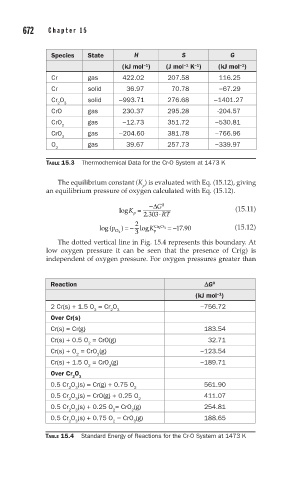
Species State H S G
(kJ mol ) (J mol K ) (kJ mol )
−1
−1
−1
−1
Cr gas 422.02 207.58 116.25
Cr solid 36.97 70.78 −67.29
Cr O solid −993.71 276.68 −1401.27
2 3
CrO gas 230.37 295.28 -204.57
CrO gas −12.73 351.72 −530.81
2
CrO gas −204.60 381.78 −766.96
3
O gas 39.67 257.73 −339.97
2
TABLE 15.3 Thermochemical Data for the Cr-O System at 1473 K
The equilibrium constant (K ) is evaluated with Eq. (15.12), giving
p
an equilibrium pressure of oxygen calculated with Eq. (15.12).
−∆
G
logK = 2 303⋅ 0 RT (15.11)
p
.
log(p ) = − 2 logK Cr O 3 = − 17.90 (15.12)
2
O 2 3 p
The dotted vertical line in Fig. 15.4 represents this boundary. At
low oxygen pressure it can be seen that the presence of Cr(g) is
independent of oxygen pressure. For oxygen pressures greater than
Reaction DG 0
(kJ mol )
−1
2 Cr(s) + 1.5 O = Cr O −756.72
2 2 3
Over Cr(s)
Cr(s) = Cr(g) 183.54
Cr(s) + 0.5 O = CrO(g) 32.71
2
Cr(s) + O = CrO (g) −123.54
2 2
Cr(s) + 1.5 O = CrO (g) −189.71
2 3
Over Cr O
2 3
0.5 Cr O (s) = Cr(g) + 0.75 O 561.90
2 3 2
0.5 Cr O (s) = CrO(g) + 0.25 O 411.07
2 3 2
0.5 Cr O (s) + 0.25 O = CrO (g) 254.81
2 3 2 2
0.5 Cr O (s) + 0.75 O = CrO (g) 188.65
2 3 2 3
TABLE 15.4 Standard Energy of Reactions for the Cr-O System at 1473 K