Page 720 - Corrosion Engineering Principles and Practice
P. 720
672 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 673
the Cr/Cr O equilibrium, the Cr(g) vapor pressure may be obtained
2
3
from the equilibrium expressed in Eq. (15.13).
0.5 Cr O (s) = Cr(g) 0.75 O (g) (15.13)
+
3
2
2
The other lines in Fig. 15.4 are obtained by using similar
equilibrium equations (Table 15.4). The vapor equilibria presented in
Fig. 15.4 show that significant Cr(g) vapor pressures are developed at
low-oxygen partial pressure (e.g., at the alloy-scale interface of a
Cr O -forming alloy) but that a much larger pressure of CrO (g)
3
3
2
develops at high-oxygen partial pressure. This high CrO (g) pressure
3
is responsible for the thinning of Cr O scales by vapor losses during
3
2
exposure to oxygen-rich environments.
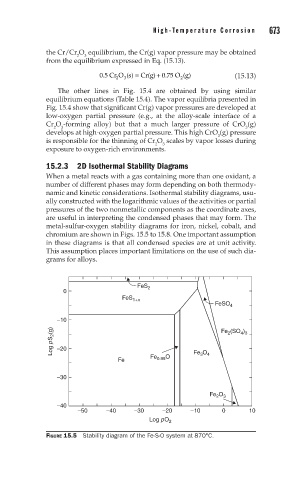
15.2.3 2D Isothermal Stability Diagrams
When a metal reacts with a gas containing more than one oxidant, a
number of different phases may form depending on both thermody-
namic and kinetic considerations. Isothermal stability diagrams, usu-
ally constructed with the logarithmic values of the activities or partial
pressures of the two nonmetallic components as the coordinate axes,
are useful in interpreting the condensed phases that may form. The
metal-sulfur-oxygen stability diagrams for iron, nickel, cobalt, and
chromium are shown in Figs. 15.5 to 15.8. One important assumption
in these diagrams is that all condensed species are at unit activity.
This assumption places important limitations on the use of such dia-
grams for alloys.
FeS 2
0
FeS 1+x
FeSO 4
–10
Log pS 2 (g) –20 Fe (SO )
4 3
2
3
Fe Fe 0.95 O Fe O 4
–30
Fe O 3
2
–40
–50 –40 –30 –20 –10 0 10
Log pO 2
FIGURE 15.5 Stability diagram of the Fe-S-O system at 870°C.