Page 723 - Corrosion Engineering Principles and Practice
P. 723
676 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 677
15.3.1 Scale as a Diffusion Barrier
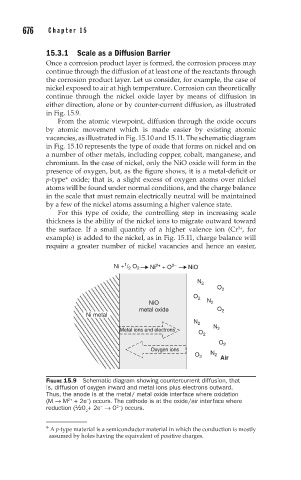
Once a corrosion product layer is formed, the corrosion process may
continue through the diffusion of at least one of the reactants through
the corrosion product layer. Let us consider, for example, the case of
nickel exposed to air at high temperature. Corrosion can theoretically
continue through the nickel oxide layer by means of diffusion in
either direction, alone or by counter-current diffusion, as illustrated
in Fig. 15.9.
From the atomic viewpoint, diffusion through the oxide occurs
by atomic movement which is made easier by existing atomic
vacancies, as illustrated in Fig. 15.10 and 15.11. The schematic diagram
in Fig. 15.10 represents the type of oxide that forms on nickel and on
a number of other metals, including copper, cobalt, manganese, and
chromium. In the case of nickel, only the NiO oxide will form in the
presence of oxygen, but, as the figure shows, it is a metal-deficit or
p-type* oxide; that is, a slight excess of oxygen atoms over nickel
atoms will be found under normal conditions, and the charge balance
in the scale that must remain electrically neutral will be maintained
by a few of the nickel atoms assuming a higher valence state.
For this type of oxide, the controlling step in increasing scale
thickness is the ability of the nickel ions to migrate outward toward
the surface. If a small quantity of a higher valence ion (Cr , for
3+
example) is added to the nickel, as in Fig. 15.11, charge balance will
require a greater number of nickel vacancies and hence an easier,
1
2+
Ni + / O Ni + O 2– NiO
2
2
N 2
O 2
O 2
NiO N 2
metal oxide O 2
Ni metal
N 2
Metal ions and electrons O 2 N 2
O 2
Oxygen ions
O 2 N 2 Air
FIGURE 15.9 Schematic diagram showing countercurrent diffusion, that
is, diffusion of oxygen inward and metal ions plus electrons outward.
Thus, the anode is at the metal/ metal oxide interface where oxidation
(M → M + 2e ) occurs. The cathode is at the oxide/air interface where
2+
−
2−
reduction (½O + 2e → O ) occurs.
−
2
* A p-type material is a semiconductor material in which the conduction is mostly
assumed by holes having the equivalent of positive charges.