Page 728 - Corrosion Engineering Principles and Practice
P. 728
680 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 681
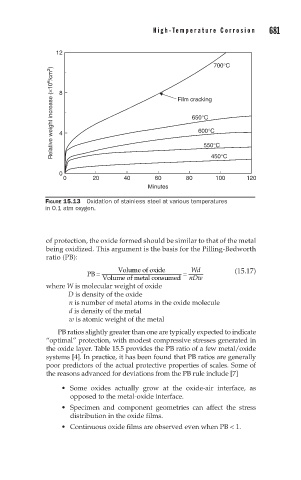
12
700°C
Relative weight increase (×10 6 /cm 2 ) 8 4 Film cracking
650°C
600°C
450°C
0 550°C
0 20 40 60 80 100 120
Minutes
FIGURE 15.13 Oxidation of stainless steel at various temperatures
in 0.1 atm oxygen.
of protection, the oxide formed should be similar to that of the metal
being oxidized. This argument is the basis for the Pilling-Bedworth
ratio (PB):
PB = Volume of oxide = W Wd (15.17)
Volume of metal consumed nDw
where W is molecular weight of oxide
D is density of the oxide
n is number of metal atoms in the oxide molecule
d is density of the metal
w is atomic weight of the metal
PB ratios slightly greater than one are typically expected to indicate
“optimal” protection, with modest compressive stresses generated in
the oxide layer. Table 15.5 provides the PB ratio of a few metal/oxide
systems [4]. In practice, it has been found that PB ratios are generally
poor predictors of the actual protective properties of scales. Some of
the reasons advanced for deviations from the PB rule include [7]
• Some oxides actually grow at the oxide-air interface, as
opposed to the metal-oxide interface.
• Specimen and component geometries can affect the stress
distribution in the oxide films.
• Continuous oxide films are observed even when PB < 1.