Page 729 - Corrosion Engineering Principles and Practice
P. 729
682 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 683
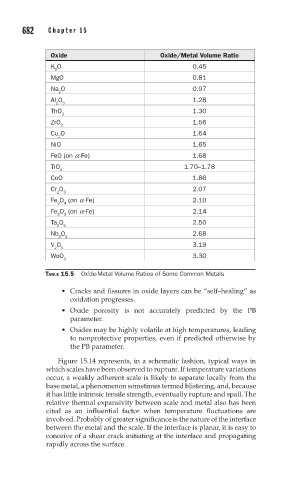
Oxide Oxide/Metal Volume Ratio
K O 0.45
2
MgO 0.81
Na O 0.97
2
Al O 1.28
2 3
ThO 1.30
2
ZrO 1.56
2
Cu O 1.64
2
NiO 1.65
FeO (on a -Fe) 1.68
TiO 1.70–1.78
2
CoO 1.86
Cr O 2.07
2 3
Fe O (on a -Fe) 2.10
3 4
Fe O (on a -Fe) 2.14
2 3
Ta O 5 2.50
2
Nb O 2.68
2 5
V O 3.19
2 5
WoO 3.30
3
TABLE 15.5 Oxide-Metal Volume Ratios of Some Common Metals
• Cracks and fissures in oxide layers can be “self–healing” as
oxidation progresses.
• Oxide porosity is not accurately predicted by the PB
parameter.
• Oxides may be highly volatile at high temperatures, leading
to nonprotective properties, even if predicted otherwise by
the PB parameter.
Figure 15.14 represents, in a schematic fashion, typical ways in
which scales have been observed to rupture. If temperature variations
occur, a weakly adherent scale is likely to separate locally from the
base metal, a phenomenon sometimes termed blistering, and, because
it has little intrinsic tensile strength, eventually rupture and spall. The
relative thermal expansivity between scale and metal also has been
cited as an influential factor when temperature fluctuations are
involved. Probably of greater significance is the nature of the interface
between the metal and the scale. If the interface is planar, it is easy to
conceive of a shear crack initiating at the interface and propagating
rapidly across the surface.