Page 731 - Corrosion Engineering Principles and Practice
P. 731
684 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 685
alloys and gas mixtures. For practical problems, both the corrosive envi-
ronment and the high temperature corrosion mechanism(s) have to be
understood. In the introduction, it was pointed out that several high-
temperature corrosion mechanisms exist. Although considerable data is
available from the literature for high-temperature corrosion in air, for
low-sulfur flue gases and for some other common refinery and petro-
chemical environments, small variations in the composition of a process
stream or in operating conditions can cause markedly different corro-
sion rates. Therefore, the most reliable basis for material selection is op-
erating experience from similar plants and environments or from pilot
plant evaluation [8].
When considering specific alloys for high-temperature service, it
is imperative to consider other properties besides the corrosion
resistance. It would be futile, for example, to select a stainless steel
with high-corrosion resistance for an application in which strength
requirements could not be met. In general, austenitic stainless steels
are substantially stronger than ferritic stainless steels at high
temperatures, as indicated by a comparison of stress rupture
properties (Fig. 15.15) and creep properties (Fig. 15.16) [3].
Some specific corrosion mechanisms are described in more detail
in the following sections. A brief description of the alloys mentioned
in these sections is provided in Table 15.6.
15.4.1 Oxidation
Oxidation is generally described as the most commonly encoun-
tered form of high-temperature corrosion. However, the oxidation
process itself is not always detrimental. In fact, most corrosion and
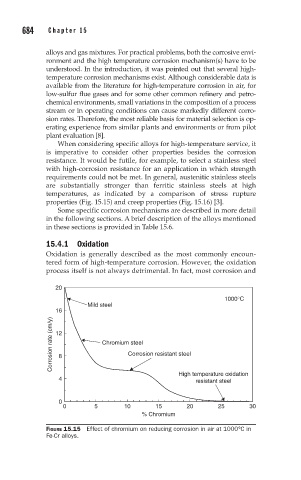
20
1000°C
Mild steel
16
Corrosion rate (cm/y) 12 Chromium steel
Corrosion resistant steel
8
4 High temperature oxidation
resistant steel
0
0 5 10 15 20 25 30
% Chromium
FIGURE 15.15 Effect of chromium on reducing corrosion in air at 1000°C in
Fe-Cr alloys.