Page 734 - Corrosion Engineering Principles and Practice
P. 734
686 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 687
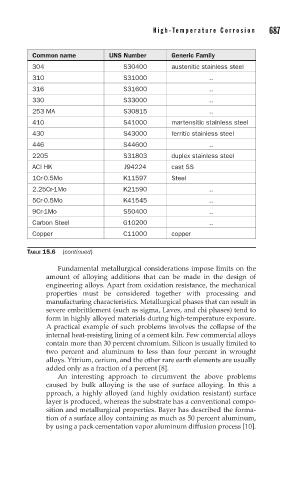
Common name UNS Number Generic Family
304 S30400 austenitic stainless steel
310 S31000 ..
316 S31600 ..
330 S33000 ..
253 MA S30815 ..
410 S41000 martensitic stainless steel
430 S43000 ferritic stainless steel
446 S44600 ..
2205 S31803 duplex stainless steel
ACI HK J94224 cast SS
1Cr-0.5Mo K11597 Steel
2.25Cr-1Mo K21590 ..
5Cr-0.5Mo K41545 ..
9Cr-1Mo S50400 ..
Carbon Steel G10200 ..
Copper C11000 copper
TABLE 15.6 (continued)
Fundamental metallurgical considerations impose limits on the
amount of alloying additions that can be made in the design of
engineering alloys. Apart from oxidation resistance, the mechanical
properties must be considered together with processing and
manufacturing characteristics. Metallurgical phases that can result in
severe embrittlement (such as sigma, Laves, and chi phases) tend to
form in highly alloyed materials during high-temperature exposure.
A practical example of such problems involves the collapse of the
internal heat-resisting lining of a cement kiln. Few commercial alloys
contain more than 30 percent chromium. Silicon is usually limited to
two percent and aluminum to less than four percent in wrought
alloys. Yttrium, cerium, and the other rare earth elements are usually
added only as a fraction of a percent [8].
An interesting approach to circumvent the above problems
caused by bulk alloying is the use of surface alloying. In this a
pproach, a highly alloyed (and highly oxidation resistant) surface
layer is produced, whereas the substrate has a conventional compo-
sition and metallurgical properties. Bayer has described the forma-
tion of a surface alloy containing as much as 50 percent aluminum,
by using a pack cementation vapor aluminum diffusion process [10].