Page 736 - Corrosion Engineering Principles and Practice
P. 736
688 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 689
in Cr or Al, whose oxides are stabilized by increasing O levels. Alloys,
2
which generally exhibit increased oxidation rates as the O
2
concentration increase, are S30400, S41000, and S44600 stainless steels
and 9Cr-1Mo, Incoloy DS, alloys 617, and 253MA. These alloys tend to
form poor oxide scales [2].
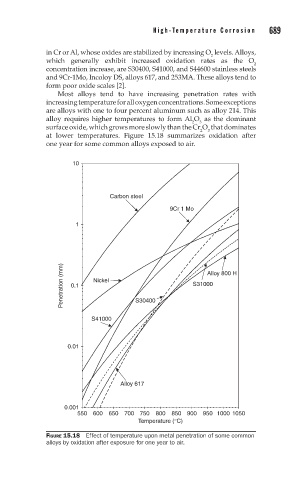
Most alloys tend to have increasing penetration rates with
increasing temperature for all oxygen concentrations. Some exceptions
are alloys with one to four percent aluminum such as alloy 214. This
alloy requires higher temperatures to form Al O as the dominant
3
2
surface oxide, which grows more slowly than the Cr O that dominates
2
3
at lower temperatures. Figure 15.18 summarizes oxidation after
one year for some common alloys exposed to air.
10
Carbon steel
9Cr 1 Mo
1
Penetration (mm) 0.1 Nickel S30400 S31000
Alloy 800 H
S41000
0.01
Alloy 617
0.001
550 600 650 700 750 800 850 900 950 1000 1050
Temperature (°C)
FIGURE 15.18 Effect of temperature upon metal penetration of some common
alloys by oxidation after exposure for one year to air.