Page 732 - Corrosion Engineering Principles and Practice
P. 732
684 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 685
15
12
1200°C
Corrosion rate (cm/y) 9 6
1095°C
3 980°C
870°C
0
0 10 20 30 40 50 60 70
% Nickel
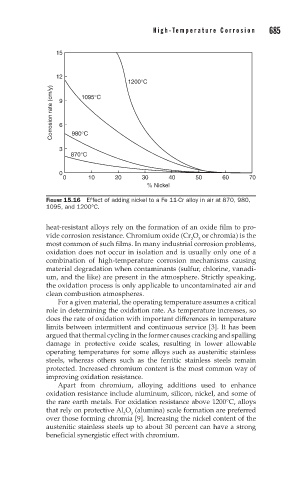
FIGURE 15.16 Effect of adding nickel to a Fe 11-Cr alloy in air at 870, 980,
1095, and 1200°C.
heat-resistant alloys rely on the formation of an oxide film to pro-
vide corrosion resistance. Chromium oxide (Cr O or chromia) is the
3
2
most common of such films. In many industrial corrosion problems,
oxidation does not occur in isolation and is usually only one of a
combination of high-temperature corrosion mechanisms causing
material degradation when contaminants (sulfur, chlorine, vanadi-
um, and the like) are present in the atmosphere. Strictly speaking,
the oxidation process is only applicable to uncontaminated air and
clean combustion atmospheres.
For a given material, the operating temperature assumes a critical
role in determining the oxidation rate. As temperature increases, so
does the rate of oxidation with important differences in temperature
limits between intermittent and continuous service [3]. It has been
argued that thermal cycling in the former causes cracking and spalling
damage in protective oxide scales, resulting in lower allowable
operating temperatures for some alloys such as austenitic stainless
steels, whereas others such as the ferritic stainless steels remain
protected. Increased chromium content is the most common way of
improving oxidation resistance.
Apart from chromium, alloying additions used to enhance
oxidation resistance include aluminum, silicon, nickel, and some of
the rare earth metals. For oxidation resistance above 1200°C, alloys
that rely on protective Al O (alumina) scale formation are preferred
3
2
over those forming chromia [9]. Increasing the nickel content of the
austenitic stainless steels up to about 30 percent can have a strong
beneficial synergistic effect with chromium.