Page 724 - Corrosion Engineering Principles and Practice
P. 724
676 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 677
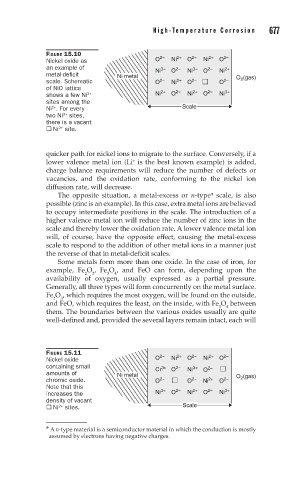
FIGURE 15.10 2– 2+ 2– 2+ 2–
Nickel oxide as O Ni O Ni O
an example of Ni 3+ O 2– Ni 3+ O 2– Ni 2+
metal-deficit Ni metal O 2 (gas)
scale. Schematic O 2– Ni 2+ O 2– O 2–
of NiO lattice 2+ 2– 2+ 2– 3+
3+
shows a few Ni Ni O Ni O Ni
sites among the
Ni . For every Scale
2+
3+
two Ni sites,
there is a vacant
® Ni site.
2+
quicker path for nickel ions to migrate to the surface. Conversely, if a
lower valence metal ion (Li is the best known example) is added,
+
charge balance requirements will reduce the number of defects or
vacancies, and the oxidation rate, conforming to the nickel ion
diffusion rate, will decrease.
The opposite situation, a metal-excess or n-type* scale, is also
possible (zinc is an example). In this case, extra metal ions are believed
to occupy intermediate positions in the scale. The introduction of a
higher valence metal ion will reduce the number of zinc ions in the
scale and thereby lower the oxidation rate. A lower valence metal ion
will, of course, have the opposite effect, causing the metal-excess
scale to respond to the addition of other metal ions in a manner just
the reverse of that in metal-deficit scales.
Some metals form more than one oxide. In the case of iron, for
example, Fe O , Fe O , and FeO can form, depending upon the
3
3
4
2
availability of oxygen, usually expressed as a partial pressure.
Generally, all three types will form concurrently on the metal surface.
Fe O , which requires the most oxygen, will be found on the outside,
2
3
and FeO, which requires the least, on the inside, with Fe O between
3
4
them. The boundaries between the various oxides usually are quite
well-defined and, provided the several layers remain intact, each will
FIGURE 15.11 2– 2+ 2– 2+ 2–
Nickel oxide O Ni O Ni O
containing small Cr 3+ O 2– Ni 3+ 2–
amounts of Ni metal O O 2 (gas)
chromic oxide. O 2– O 2– Ni 2+ O 2–
Note that this
increases the Ni 3+ O 2– Ni 2+ O 2– Ni 3+
density of vacant
® Ni sites. Scale
2+
* A n-type material is a semiconductor material in which the conduction is mostly
assumed by electrons having negative charges.