Page 31 - Distillation theory
P. 31
P1: FCH
0521820928c01 CB644-Petlyuk-v1 June 11, 2004 17:45
1.4 Phase Diagrams of Three-Component Mixtures 5
However, later in this book, we will see that if x Az = 1, it is impossible to get
1
component 1 with a high degree of purity, and if x Az = 0, it is impossible to get
1
component 2 with a high degree of purity.
1.4. Phase Diagrams of Three-Component Mixtures
Three-component mixtures represent the simplest type of multicomponent mix-
tures. The majority of multicomponent mixture peculiarities become apparent in
three-component mixtures. This is why the three-component mixtures are best
studied. Liquid–vapor equilibrium in the concentration triangle C 3 is represented
by a vector connecting a point of liquid composition with a point of equilibrium
vapor composition x → y. This vector is called a liquid–vapor tie-line. The opposite
vector y → x (vapor–liquid) is called a vapor–liquid tie-line. The tie-lines field in
the concentration triangle characterizes phase equilibrium in each of its points.
However, tie-lines can cross each other. That is why, for phase equilibrium
characteristics in the concentration space, it is convenient to use another kind of
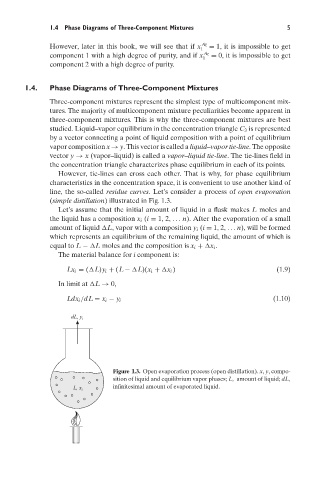
line, the so-called residue curves. Let’s consider a process of open evaporation
(simple distillation) illustrated in Fig. 1.3.
Let’s assume that the initial amount of liquid in a flask makes L moles and
the liquid has a composition x i (i = 1, 2, ... n). After the evaporation of a small
amount of liquid L, vapor with a composition y i (i = 1, 2, ... n), will be formed
which represents an equilibrium of the remaining liquid, the amount of which is
equal to L − L moles and the composition is x i + x i .
The material balance for i component is:
Lx i = ( L)y i + (L− L)(x i + x i ) (1.9)
In limit at L → 0,
Ldx i /dL = x i − y i (1.10)
dL, y
i
Figure 1.3. Open evaporation process (open distillation). x, y, compo-
sition of liquid and equilibrium vapor phases; L, amount of liquid; dL,
L, x infinitesimal amount of evaporated liquid.
i