Page 39 - Distillation theory
P. 39
P1: FCH
0521820928c01 CB644-Petlyuk-v1 June 11, 2004 17:45
13
1.7 Lines, Surfaces, and Hypersurfaces K i = K j
2 2
a) b)
α 13
α 13 213 α 12 α 23
α 231 123 α 12
23
321 123
312 α 23 4
1 α α 3 1
12
13 23 132 α α
12 12
13 α 23
3
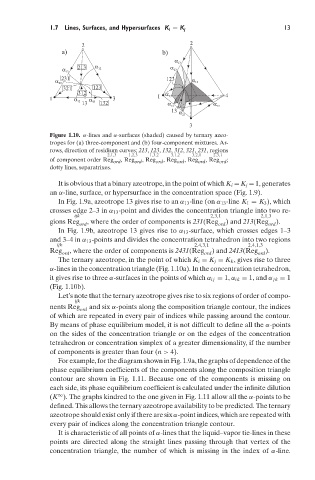
Figure 1.10. α-lines and α-surfaces (shaded) caused by ternary azeo-
tropes for (a) three-component and (b) four-component mixtures. Ar-
rows, direction of residium curves; 213, 123, 132, 312, 321, 231, regions
2,1,3 1,2,3 1,3,2 3,1,2 3,2,1 2,3,1
of component order Reg ord , Reg ord , Reg ord , Reg ord , Reg ord , Reg ord ;
dotty lines, separatrixes.
It is obvious that a binary azeotrope, in the point of which K i = K j = 1, generates
an α-line, surface, or hypersurface in the concentration space (Fig. 1.9).
In Fig. 1.9a, azeotrope 13 gives rise to an α 13 -line (on α 13 -line K 1 = K 3 ), which
crosses edge 2–3in α 13 -point and divides the concentration triangle into two re-
ijk 2,3,1 2,1,3
gions Reg , where the order of components is 231(Reg ) and 213(Reg ).
ord ord ord
In Fig. 1.9b, azeotrope 13 gives rise to α 13 -surface, which crosses edges 1–3
and 3–4in α 13 -points and divides the concentration tetrahedron into two regions
ijk 2,4,3,1 2,4,1,3
Reg , where the order of components is 2431(Reg ) and 2413(Reg ).
ord ord ord
The ternary azeotrope, in the point of which K i = K j = K k , gives rise to three
α-lines in the concentration triangle (Fig. 1.10a). In the concentration tetrahedron,
it gives rise to three α-surfaces in the points of which α ij = 1, α ik = 1, and α jk = 1
(Fig. 1.10b).
Let’s note that the ternary azeotrope gives rise to six regions of order of compo-
ijk
nents Reg ord and six α-points along the composition triangle contour, the indices
of which are repeated in every pair of indices while passing around the contour.
By means of phase equilibrium model, it is not difficult to define all the α-points
on the sides of the concentration triangle or on the edges of the concentration
tetrahedron or concentration simplex of a greater dimensionality, if the number
of components is greater than four (n > 4).
For example, for the diagram shown in Fig. 1.9a, the graphs of dependence of the
phase equilibrium coefficients of the components along the composition triangle
contour are shown in Fig. 1.11. Because one of the components is missing on
each side, its phase equilibrium coefficient is calculated under the infinite dilution
(K ). The graphs kindred to the one given in Fig. 1.11 allow all the α-points to be
∞
defined. This allows the ternary azeotrope availability to be predicted. The ternary
azeotrope should exist only if there are six α-point indices, which are repeated with
every pair of indices along the concentration triangle contour.
It is characteristic of all points of α-lines that the liquid–vapor tie-lines in these
points are directed along the straight lines passing through that vertex of the
concentration triangle, the number of which is missing in the index of α-line.