Page 137 - Electrical Installation in Hazardous Area
P. 137
Calculation of release rates and extents 103
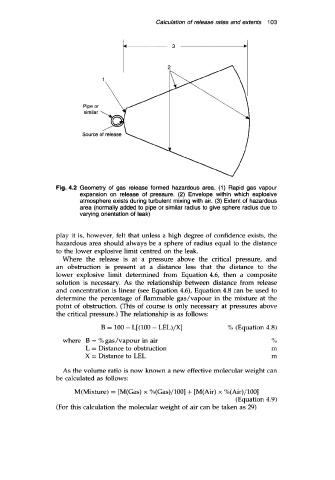
Fig. 4.2 Geometry of gas release formed hazardous area. (1) Rapid gas vapour
expansion on release of pressure. (2) Envelope within which explosive
atmosphere exists during turbulent mixing with air. (3) Extent of hazardous
area (normally added to pipe or similar radius to give sphere radius due to
varying orientation of leak)
play it is, however, felt that unless a high degree of confidence exists, the
hazardous area should always be a sphere of radius equal to the distance
to the lower explosive limit centred on the leak.
Where the release is at a pressure above the critical pressure, and
an obstruction is present at a distance less that the distance to the
lower explosive limit determined from Equation 4.6, then a composite
solution is necessary. As the relationship between distance from release
and concentration is linear (see Equation 4.6), Equation 4.8 can be used to
determine the percentage of flammable gas/vapour in the mixture at the
point of obstruction. (This of course is only necessary at pressures above
the critical pressure.) The relationship is as follows:
B = 100 - L[(100 - LEL)/X] o/o (Equation 4.8)
where B = %gas/vapour in air m
O/O
L = Distance to obstruction
X = Distance to LEL m
As the volume ratio is now known a new effective molecular weight can
be calculated as follows:
M(Mixture) = [M(Gas) x %(Gas)/100] + [M(Air) x %(Air)/100]
(Equation 4.9)
(For this calculation the molecular weight of air can be taken as 29)