Page 115 - Electrical Properties of Materials
P. 115
Quartz–halogen lamps 97
device, called the field-ion microscope, was the first in the history of science to
make individual atoms visible. Thus, just about two and a half millennia after
introducing the concept of atoms, it proved possible to see them.
6.9 The photoelectric effect
Emission of electrons owing to the incidence of electromagnetic waves is
called the photoelectric effect. The word photo (light in Greek) came into the Albert Einstein received the No-
description because the effect was first found in the visible range. Interestingly, bel Prize in 1921 for ‘his discovery
it is one of the earliest phenomena that cast serious doubts on the validity of of the law of the photoelectric ef-
classical physics and was instrumental in the birth of quantum physics. fect’. It is interesting to note that
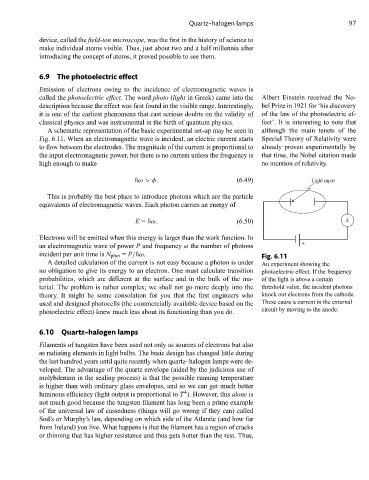
A schematic representation of the basic experimental set-up may be seen in although the main tenets of the
Fig. 6.11. When an electromagnetic wave is incident, an electric current starts Special Theory of Relativity were
to flow between the electrodes. The magnitude of the current is proportional to already proven experimentally by
the input electromagnetic power, but there is no current unless the frequency is that time, the Nobel citation made
high enough to make no mention of relativity.
ω> φ. (6.49) Light input
This is probably the best place to introduce photons which are the particle
equivalents of electromagnetic waves. Each photon carries an energy of
E = ω. (6.50) A
Electrons will be emitted when this energy is larger than the work function. In
+
an electromagnetic wave of power P and frequency ω the number of photons
incident per unit time is N phot = P/ ω. Fig. 6.11
A detailed calculation of the current is not easy because a photon is under An experiment showing the
no obligation to give its energy to an electron. One must calculate transition photoelectric effect. If the frequency
probabilities, which are different at the surface and in the bulk of the ma- of the light is above a certain
terial. The problem is rather complex; we shall not go more deeply into the threshold value, the incident photons
theory. It might be some consolation for you that the first engineers who knock out electrons from the cathode.
used and designed photocells (the commercially available device based on the These cause a current in the external
circuit by moving to the anode.
photoelectric effect) knew much less about its functioning than you do.
6.10 Quartz–halogen lamps
Filaments of tungsten have been used not only as sources of electrons but also
as radiating elements in light bulbs. The basic design has changed little during
the last hundred years until quite recently when quartz–halogen lamps were de-
veloped. The advantage of the quartz envelope (aided by the judicious use of
molybdenum in the sealing process) is that the possible running temperature
is higher than with ordinary glass envelopes, and so we can get much better
4
luminous efficiency (light output is proportional to T ). However, this alone is
not much good because the tungsten filament has long been a prime example
of the universal law of cussedness (things will go wrong if they can) called
Sod’s or Murphy’s law, depending on which side of the Atlantic (and how far
from Ireland) you live. What happens is that the filament has a region of cracks
or thinning that has higher resistance and thus gets hotter than the rest. Thus,