Page 116 - Electrical Properties of Materials
P. 116
98 The free electron theory of metals
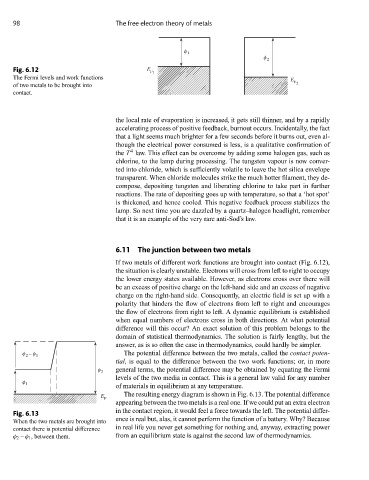
φ 1
φ 2
Fig. 6.12 E F 1
The Fermi levels and work functions E
of two metals to be brought into F 2
contact.
the local rate of evaporation is increased, it gets still thinner, and by a rapidly
accelerating process of positive feedback, burnout occurs. Incidentally, the fact
that a light seems much brighter for a few seconds before it burns out, even al-
though the electrical power consumed is less, is a qualitative confirmation of
4
the T law. This effect can be overcome by adding some halogen gas, such as
chlorine, to the lamp during processing. The tungsten vapour is now conver-
ted into chloride, which is sufficiently volatile to leave the hot silica envelope
transparent. When chloride molecules strike the much hotter filament, they de-
compose, depositing tungsten and liberating chlorine to take part in further
reactions. The rate of depositing goes up with temperature, so that a ‘hot spot’
is thickened, and hence cooled. This negative feedback process stabilizes the
lamp. So next time you are dazzled by a quartz–halogen headlight, remember
that it is an example of the very rare anti-Sod’s law.
6.11 The junction between two metals
If two metals of different work functions are brought into contact (Fig. 6.12),
the situation is clearly unstable. Electrons will cross from left to right to occupy
the lower energy states available. However, as electrons cross over there will
be an excess of positive charge on the left-hand side and an excess of negative
charge on the right-hand side. Consequently, an electric field is set up with a
polarity that hinders the flow of electrons from left to right and encourages
the flow of electrons from right to left. A dynamic equilibrium is established
when equal numbers of electrons cross in both directions. At what potential
difference will this occur? An exact solution of this problem belongs to the
domain of statistical thermodynamics. The solution is fairly lengthy, but the
answer, as is so often the case in thermodynamics, could hardly be simpler.
_ The potential difference between the two metals, called the contact poten-
φ 2 φ 1
tial, is equal to the difference between the two work functions; or, in more
general terms, the potential difference may be obtained by equating the Fermi
φ 2
levels of the two media in contact. This is a general law valid for any number
φ 1
of materials in equilibrium at any temperature.
E The resulting energy diagram is shown in Fig. 6.13. The potential difference
F
appearing between the two metals is a real one. If we could put an extra electron
in the contact region, it would feel a force towards the left. The potential differ-
Fig. 6.13
ence is real but, alas, it cannot perform the function of a battery. Why? Because
When the two metals are brought into
contact there is potential difference in real life you never get something for nothing and, anyway, extracting power
φ 2 – φ 1 , between them. from an equilibrium state is against the second law of thermodynamics.