Page 155 - Electrical Properties of Materials
P. 155
III–V and II–VI compounds 137
excitations, that is electrons can be excited into the conduction band. The only
difference relative to Ge and Si is that III–V compounds have an ionic contribu-
tion to the bonding as well. This is not particularly surprising. We mentioned in
Chapter 5 that the ionic bond of NaCl comes about because the one outer elec-
tron of sodium is happy to join the seven outer electrons of chlorine to make up
a completed ring—so it is easy to see that, with Ga having three outer electrons
and As having five outer electrons, they will also strike up a companionship in
order to complete the ring.
Which are the most important III–V materials? The oldest one, and Note, however, that a III–V ionic
technologically the best developed, is GaAs, to which serious attention has bond is weaker than a I–VII
been paid since the middle of the 1950s and which has been the preferred ma- ionic bond.
terial for a host of devices. Why? One might expect that arsenic was the last
thing anyone in any laboratory would have wanted to work on. However, some
of the rivals, for example AlSb, fell by the wayside because of a tendency to
decompose quickly, and, most importantly, GaAs was the material that offered
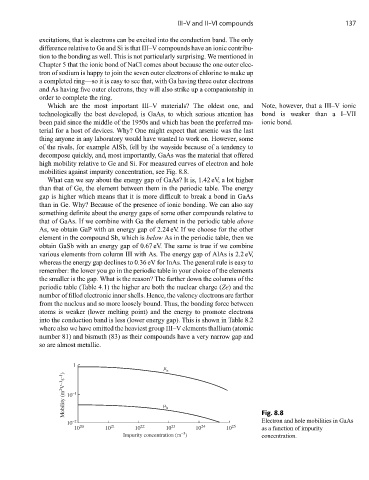
high mobility relative to Ge and Si. For measured curves of electron and hole
mobilities against impurity concentration, see Fig. 8.8.
What can we say about the energy gap of GaAs? It is, 1.42 eV, a lot higher
than that of Ge, the element between them in the periodic table. The energy
gap is higher which means that it is more difficult to break a bond in GaAs
than in Ge. Why? Because of the presence of ionic bonding. We can also say
something definite about the energy gaps of some other compounds relative to
that of GaAs. If we combine with Ga the element in the periodic table above
As, we obtain GaP with an energy gap of 2.24 eV. If we choose for the other
element in the compound Sb, which is below As in the periodic table, then we
obtain GaSb with an energy gap of 0.67 eV. The same is true if we combine
various elements from column III with As. The energy gap of AlAs is 2.2 eV,
whereas the energy gap declines to 0.36 eV for InAs. The general rule is easy to
remember: the lower you go in the periodic table in your choice of the elements
the smaller is the gap. What is the reason? The farther down the columns of the
periodic table (Table 4.1) the higher are both the nuclear charge (Ze) and the
number of filled electronic inner shells. Hence, the valency electrons are farther
from the nucleus and so more loosely bound. Thus, the bonding force between
atoms is weaker (lower melting point) and the energy to promote electrons
into the conduction band is less (lower energy gap). This is shown in Table 8.2
where also we have omitted the heaviest group III–V elements thallium (atomic
number 81) and bismuth (83) as their compounds have a very narrow gap and
so are almost metallic.
1
e
Mobility (m 2 V –1 s –1 ) 10 –1 h
Electron and hole mobilities in GaAs
10 –2 Fig. 8.8
10 20 10 21 10 22 10 23 10 24 10 25 as a function of impurity
–3
Impurity concentration (m ) concentration.