Page 158 - Electrical Properties of Materials
P. 158
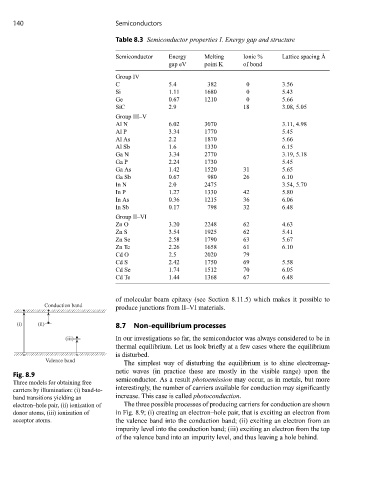
140 Semiconductors
Table 8.3 Semiconductor properties I. Energy gap and structure
Semiconductor Energy Melting Ionic % Lattice spacing Å
gap eV point K of bond
Group IV
C 5.4 382 0 3.56
Si 1.11 1680 0 5.43
Ge 0.67 1210 0 5.66
SiC 2.9 18 3.08, 5.05
Group III–V
Al N 6.02 3070 3.11, 4.98
Al P 3.34 1770 5.45
Al As 2.2 1870 5.66
Al Sb 1.6 1330 6.15
Ga N 3.34 2770 3.19, 5.18
Ga P 2.24 1730 5.45
Ga As 1.42 1520 31 5.65
Ga Sb 0.67 980 26 6.10
In N 2.0 2475 3.54, 5.70
In P 1.27 1330 42 5.80
In As 0.36 1215 36 6.06
In Sb 0.17 798 32 6.48
Group II–VI
Zn O 3.20 2248 62 4.63
Zn S 3.54 1925 62 5.41
Zn Se 2.58 1790 63 5.67
Zn Te 2.26 1658 61 6.10
Cd O 2.5 2020 79
Cd S 2.42 1750 69 5.58
Cd Se 1.74 1512 70 6.05
Cd Te 1.44 1368 67 6.48
of molecular beam epitaxy (see Section 8.11.5) which makes it possible to
Conduction band produce junctions from II–VI materials.
(i) (ii) 8.7 Non-equilibrium processes
(iii) In our investigations so far, the semiconductor was always considered to be in
thermal equilibrium. Let us look briefly at a few cases where the equilibrium
is disturbed.
Valence band
The simplest way of disturbing the equilibrium is to shine electromag-
netic waves (in practice these are mostly in the visible range) upon the
Fig. 8.9
Three models for obtaining free semiconductor. As a result photoemission may occur, as in metals, but more
carriers by illumination: (i) band-to- interestingly, the number of carriers available for conduction may significantly
band transitions yielding an increase. This case is called photoconduction.
electron–hole pair, (ii) ionization of The three possible processes of producing carriers for conduction are shown
donor atoms, (iii) ionization of in Fig. 8.9; (i) creating an electron–hole pair, that is exciting an electron from
acceptor atoms. the valence band into the conduction band; (ii) exciting an electron from an
impurity level into the conduction band; (iii) exciting an electron from the top
of the valence band into an impurity level, and thus leaving a hole behind.