Page 323 - Electrical Properties of Materials
P. 323
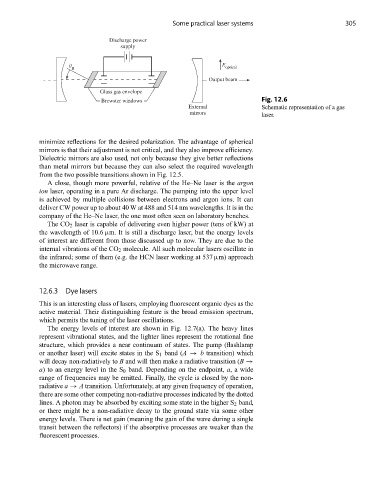
Some practical laser systems 305
Discharge power
supply
E
B optical
Output beam
Glass gas envelope
Brewster windows Fig. 12.6
External Schematic representation of a gas
mirrors laser.
minimize reflections for the desired polarization. The advantage of spherical
mirrors is that their adjustment is not critical, and they also improve efficiency.
Dielectric mirrors are also used, not only because they give better reflections
than metal mirrors but because they can also select the required wavelength
from the two possible transitions shown in Fig. 12.5.
A close, though more powerful, relative of the He–Ne laser is the argon
ion laser, operating in a pure Ar discharge. The pumping into the upper level
is achieved by multiple collisions between electrons and argon ions. It can
deliver CW power up to about 40 W at 488 and 514 nm wavelengths. It is in the
company of the He–Ne laser, the one most often seen on laboratory benches.
The CO 2 laser is capable of delivering even higher power (tens of kW) at
the wavelength of 10.6 μm. It is still a discharge laser, but the energy levels
of interest are different from those discussed up to now. They are due to the
internal vibrations of the CO 2 molecule. All such molecular lasers oscillate in
the infrared; some of them (e.g. the HCN laser working at 537 μm) approach
the microwave range.
12.6.3 Dye lasers
This is an interesting class of lasers, employing fluorescent organic dyes as the
active material. Their distinguishing feature is the broad emission spectrum,
which permits the tuning of the laser oscillations.
The energy levels of interest are shown in Fig. 12.7(a). The heavy lines
represent vibrational states, and the lighter lines represent the rotational fine
structure, which provides a near continuum of states. The pump (flashlamp
or another laser) will excite states in the S 1 band (A → b transition) which
will decay non-radiatively to B and will then make a radiative transition (B →
a) to an energy level in the S 0 band. Depending on the endpoint, a,awide
range of frequencies may be emitted. Finally, the cycle is closed by the non-
radiative a → A transition. Unfortunately, at any given frequency of operation,
there are some other competing non-radiative processes indicated by the dotted
lines. A photon may be absorbed by exciting some state in the higher S 2 band,
or there might be a non-radiative decay to the ground state via some other
energy levels. There is net gain (meaning the gain of the wave during a single
transit between the reflectors) if the absorptive processes are weaker than the
fluorescent processes.