Page 268 - Elements of Chemical Reaction Engineering 3rd Edition
P. 268
240 Collection and Analysis of Rate Data Chap. 5
1.4 mrnHg/min. By various plotting or numerical analysis techniques relating
- yA0 to C,, , we can obtain the appropriate rate law. If the rate law is in the form
- TAO = kcio
the slope of the plot of In (- versus In C,, will give the reaction order a.
i",,)
Example 5-3 Method of Initial Rates in Solid-Liquid Dissolution Kinetics
The dissolution of dolomite, calcium magnesium carbonate, in hydrochloric acid is
a reaction of particular importance in the acid stimulation of dolomite oil reservoirs:
The oil is contained in pore space of the carbonate material and must flow through
the small pores to reach the well bore. In matrix stimulation, HC1 is injected into a
well bore to dissolve the porous carbonate matrix. By dissolving the solid carbonate
the pores will increase in size, and the oil and gas will be able to flow out at faster
rates, thereby increasing the productivity of the welL5 The dissolution reaction is
4HC: + CaMg(C0,),+Mg2+ + Ca2+ + 4C1- + 2C0, + 2H20
The concentration of HCl at various times was determined from atomic absorption
spectrophotometer measurements of the calcium and magnesium ions.
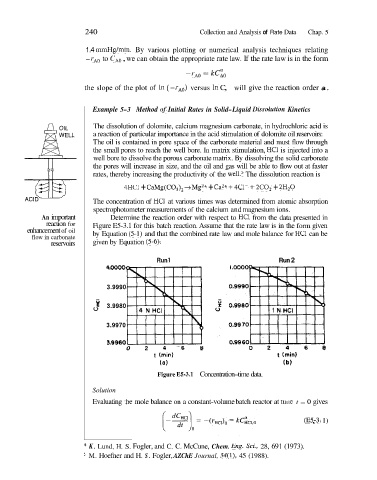
An important Determine the reaction order with respect to HC1 from the data presented in
reaction for Figure E5-3.1 for this batch reaction. Assume that the rate law is in the form given
enhancement of oil by Equation (5-1) and that the combined rate law and mole balance for HC1 can be
flow in carbonate
reservoirs given by Equation (5-6).
Run 1 Run 2
4.0000
3.9990
E) 3.9980
0
3.9970
0 2 4'6 8 0 2 4 6 8
t (rninl t (mid
(01 (b)
Figure E5-3.1 Concentration-time data.
Solution
Evaluating ,he mole balance on a constant-volume batch reactor at tiilie t = 0 gives
(E5;3: 1)
K. Lund, H. S. Fogler, and C. C. McCune, Chem. Eng. Sci., 28, 691 (1973).
M. Hoefner and H. S. Fogler, AZChE Journal, 34(1), 45 (1988).