Page 264 - Elements of Chemical Reaction Engineering 3rd Edition
P. 264
236 Collection and Analysis of Rate Data Chap. 5
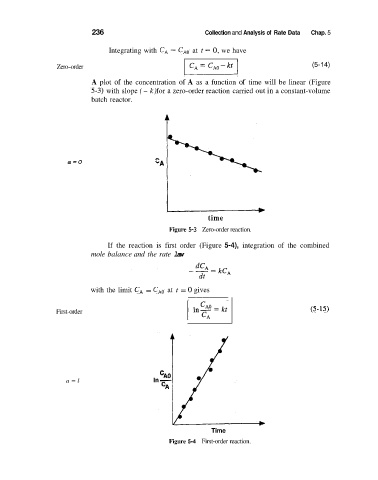
Integrating with C, = CA0 at t = 0, we have
Zero-order (5-14)
A plot of the concentration of A as a function of time will be linear (Figure
'5-3) with slope (- k) for a zero-order reaction carried out in a constant-volume
batch reactor.
a=O
time
Figure 5-3 Zero-order reaction.
If the reaction is first order (Figure 5-4), integration of the combined
mole balance and the rate law
with the limit C, = C,, at t = 0 gives
7
First-order (5-15)
a=l
Time
Figure 5-4 First-order reaction.