Page 277 - Elements of Chemical Reaction Engineering 3rd Edition
P. 277
Sec. 5.4 Differential Reactors 249
We will see in Chapter 10 that this combination and similar rate laws whic!h have
reactant. concentrations (or partial pressures) in the numerator and denominator are
common in heterogeneous catalysis.
Let’s see if the resulting rate law (E5-4.8) is qualitatively consistent with the
rate observed.
1. For condition I: At low P4, (b(PHJP24 1) and Equation (E5-4.8) reduces to
I 81 (E5-4.9)
‘CH, .- pH2
Equation (E5-4.9) is consistent with the trend in comparing runs 4 and 5.
2. For condition 2: At high PH2, b((PH2)”% 1) and Equation (E5-4.8) reduces to
(E5-4.10)
where flz > PI. Equation (E5-4.10) is consistent with the trends in comparing
runs 5 and 6.
Theoretical considerations of the type to be discussed in Chapter 10 predict
that if the ratelimiting step in the overall reaction is the reaction between atomic
hydrogen absorbed on the nickel surface and CO in the gas phase, then the rate law
will be in the form
aPcop~22
(E5-4.11)
=
This rate law is qualitatively consistent with experimental observations. To obtain
the parameter a and b, we rearrange Equation (E5-4.11) in the form
(E5-4.12)
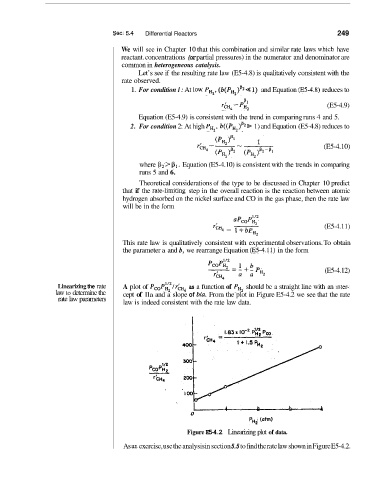
Linearizing the rate A plot of Pc0Pg/i& as a function of PHz should be a straight line with an mter-
law to determine the cept of lla and a slope of bla. From the plot in Figure E5-4.2 we see that the rate
rate law parameters
law is indeed consistent with the rate law data.
1
2
3
1
4
0
PHz (otrn)
Figure E5-4.2 Linearizing plot of data.
As ari exercise, use the analysis in section 5.5 to find the rate law shown in Figure E5-4.2.